Search found 16 matches
- Fri Mar 17, 2017 1:19 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Excellence in Chemistry Award 2015-16
- Replies: 6
- Views: 1534
Re: Excellence in Chemistry Award 2015-16
Congratulations ! Hard work pays off!
- Fri Mar 17, 2017 1:17 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Details and Review Sessions Winter 2017
- Replies: 114
- Views: 27113
Re: Final Exam Details and Review Sessions Winter 2017
Hey guys! I am filming the final review session but if anyone has a tripod and could bring it that would be super helpful for the quality and the easiness of doing it! Thank you!
- Fri Mar 17, 2017 1:15 pm
- Forum: Student Social/Study Group
- Topic: Strategies for studying for the final
- Replies: 9
- Views: 1668
Re: Strategies for studying for the final
Anyone have any great online resources for Thermodynamics studying? That's where I am most rusty!
- Fri Mar 17, 2017 1:13 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 553881
Re: Saying Thank You to Dr. Lavelle
Thank you Dr. Lavelle for your hard work and dedication to our education! All the best to you!
- Fri Mar 17, 2017 1:13 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3608199
Re: Post All Chemistry Jokes Here
Name that Molecule!
(Entrance to CS 50)
(Entrance to CS 50)
- Fri Mar 17, 2017 1:11 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3608199
Re: Post All Chemistry Jokes Here
Name that Molecule! (As seen in the entrance to CS 50)
- Sun Feb 12, 2017 10:00 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: C versus R
- Replies: 1
- Views: 405
C versus R
Why in the equation with a change in volume do we use the Gas Constant but when we calculate Entropy with a change in Temperature we use C? Just curious and for some reason cannot seem to figure it out ...
- Sun Feb 12, 2017 9:57 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpy w/ Diagram
- Replies: 2
- Views: 701
Re: Bond Enthalpy w/ Diagram
But when we calculate the change in Bond enthalpy, we have to know which specific bonds are broken and which are not broken. That being said, I agree with the difficulty in determining which ones actually break and which ones are the same. I get messed u on counting them that way.
- Sun Feb 12, 2017 9:53 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G vs Delta G0
- Replies: 5
- Views: 7878
Re: Delta G vs Delta G0
But what is the actual difference between calculating a 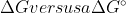 ?
?
- Sun Feb 12, 2017 9:49 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm Winter 2017
- Replies: 87
- Views: 21099
Re: Midterm Winter 2017
I would also like to know if we should know any specific derivations for the midterm. I know we haven't been tested on them in the past but there was a fairly large focus on the derivations of thermodynamics equations using the ideal gas law. Thank you!
- Sun Jan 29, 2017 8:10 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Homework 8.65
- Replies: 2
- Views: 877
Re: Homework 8.65
I am struggling with this question as well. I looked at the solutions manual but for some reason I still cannot decipher how the question was answered. The solutions do not indicate using the enthalpy of the second reaction in the problem, so I am confused as to why that value is included. I do not ...
- Sat Jan 28, 2017 7:18 pm
- Forum: Ideal Gases
- Topic: Reading the textbook
- Replies: 262
- Views: 150575
Re: Reading the textbook
I find reading the textbook very helpful. I usually like to do it while completing assigned problems- this way, I can try and learn how to solve a problem by going back and reading relevant sections instead of just trying to read the whole chapter at once without actually practicing any of the mater...
- Tue Jan 10, 2017 9:54 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Internal Energy Changes in a Closed System
- Replies: 1
- Views: 439
Internal Energy Changes in a Closed System
When solving a problem about the change in internal energy of a closed system, we use the equation (delta)U=Q-W. I was told that the equation simply means the change in internal energy combines the values of heat being added to the system and work being done by the system. Technically, this means th...
- Sat Oct 29, 2016 4:29 pm
- Forum: General Science Questions
- Topic: Rusty on High School Chem [ENDORSED]
- Replies: 347
- Views: 434962
Re: Rusty on High School Chem [ENDORSED]
Reading Fundamentals in the textbook before the ones assigned were extremely helpful as well. I felt much more prepared after I read fundamentals A-D specifically. Also, just reading the textbook provides more background knowledge that helps understanding the course reader as well.
- Sun Oct 23, 2016 1:33 pm
- Forum: *Shrodinger Equation
- Topic: Shrodinger Equation
- Replies: 4
- Views: 920
Re: Shrodinger Equation
I'm pretty sure we don't need to know how to solve Shrodinger's equation because Dr. Lavelle told us to omit Table 2.1, which is the process on how to solve for the three quantum numbers.
- Fri Oct 07, 2016 9:50 am
- Forum: Properties of Light
- Topic: Formulas need to know? [ENDORSED]
- Replies: 3
- Views: 917
Re: Formulas need to know? [ENDORSED]
I'm not 100% sure, but I'm pretty sure we will receive a formula sheet and periodic table with all the necessary equations. My TA told us that we should be given all of them, at least. As for constants, I would say to memorize them regardless because it will help you with speed on the quiz- which I ...

