Search found 30 matches
- Fri Mar 17, 2017 10:29 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Details and Review Sessions Winter 2017
- Replies: 114
- Views: 27116
Re: Final Exam Details and Review Sessions Winter 2017
Is there a link for Lavelle's review session from today?
- Sun Mar 12, 2017 2:35 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Details and Review Sessions Winter 2017
- Replies: 114
- Views: 27116
Re: Final Exam Details and Review Sessions Winter 2017
Will the final be more focused on material at the end of the quarter?
- Wed Mar 08, 2017 11:20 am
- Forum: *Free Energy of Activation vs Activation Energy
- Topic: Delta G Double Dagger, Delta H Double Dagger, Delta S Double Dagger
- Replies: 3
- Views: 5874
Re: Delta G Double Dagger, Delta H Double Dagger, Delta S Double Dagger
If this is referring to Section 4.4 in the green organic textbook, they state that all the statements they make reference the reaction in Figure 4.6, which is an SN2 reaction.
- Wed Mar 08, 2017 11:04 am
- Forum: *Alkanes
- Topic: Naming [ENDORSED]
- Replies: 93
- Views: 16688
Re: Naming [ENDORSED]
Bryan Vega 2E wrote:Would a prefix, such as "di" in dimethyl, affect the order when naming alphabetically?
no!
- Wed Mar 08, 2017 11:02 am
- Forum: *Alkanes
- Topic: Naming [ENDORSED]
- Replies: 93
- Views: 16688
Re: Naming [ENDORSED]
Will the order always be in alphabetical order when naming the structures ? Sometimes it can vary if there's a "substituent in a substituent" type of situation (i.e. IUPAC naming for isopropyl branch would be 2-methylpropyl which would be included amongst other substituents if applicable)...
- Wed Mar 01, 2017 12:45 pm
- Forum: *Organic Reaction Mechanisms in General
- Topic: Drawing Reaction Profiles
- Replies: 1
- Views: 539
Drawing Reaction Profiles
For a multistep reaction profile where we are estimating it for each step, how can we know how high/low/wide to draw each step?
- Fri Feb 24, 2017 3:07 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm Winter 2017
- Replies: 87
- Views: 21102
Re: Midterm Winter 2017
Sebastian wrote:If we weren't able to make it to lecture on Wednesday, what is another option to obtain our midterm?
I would email your TA to ask if they can bring it to office hours or your next discussion
- Fri Feb 24, 2017 3:06 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Fall 2016 Finals Still Available?
- Replies: 5
- Views: 1269
Re: Fall 2016 Finals Still Available?
They're available at the Information office (Young 3034)! I think they open at 7AM.
- Wed Feb 22, 2017 9:25 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm Winter 2017
- Replies: 87
- Views: 21102
Re: Midterm Winter 2017
Have the solutions been posted anywhere yet?
- Sun Feb 19, 2017 4:00 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Bruincast Audio Problem
- Replies: 16
- Views: 2922
Re: Bruincast Audio Problem
Just a note: Chrome does work for the audio bruincast (normal or iPod/iPad version), but you'll have to use another browser if you want to see the video!
- Wed Feb 15, 2017 11:27 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Midterm Winter 2013 problem 5B
- Replies: 10
- Views: 1812
Re: Midterm Winter 2013 problem 5B
Chem Mod also posted a response on another post saying that larger molecules with more electrons contribute more entropy because (basically) there are more total particles to be knocked around and cause disorder.
- Mon Feb 13, 2017 4:50 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Reversibility
- Replies: 2
- Views: 641
Re: Reversibility
I was in a UA review session and he said that usually the adiabatic (q=0) is irreversible--just another note!
- Mon Feb 13, 2017 4:42 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: H2O in Galvanic Cell Diagram
- Replies: 1
- Views: 1524
Re: H2O in Galvanic Cell Diagram
Definitely in acidic solution and basic solution reactions. They're not included in the cell diagrams because technically with aqueous solutions, they are by definition a solute with water so it'd be redundant to add water.
- Thu Feb 09, 2017 9:04 pm
- Forum: Balancing Redox Reactions
- Topic: Homework 14.15 (a)
- Replies: 1
- Views: 474
Homework 14.15 (a)
Instructions: "Write the half-reaction and devise a galvanic cell (write a cell diagram) to study each of the following reactions: (a) AgBr_{(s)} \rightleftharpoons Ag^+_{(aq)} + Br^-_{(aq)} , a solubility equilibrium" I don't understand where to draw the two half-r...
- Mon Jan 30, 2017 12:21 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Question 8.77
- Replies: 1
- Views: 508
Question 8.77
For this problem, it can be calculated that the energy of the unhybridized carbon bonds = 2880 kJ while the hybridized bonds = 3108 kJ. Why is the higher energy more stable in this case? Even with consideration that these bonds are the ones formed (therefore negative), the absolute energy is higher ...
- Mon Jan 30, 2017 12:04 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Question 8.77
- Replies: 1
- Views: 463
Re: Question 8.77
They're basically the unhybridized Lewis structures (being asked to compare to hybridized).
- Mon Jan 23, 2017 12:42 am
- Forum: Phase Changes & Related Calculations
- Topic: delta u sign
- Replies: 2
- Views: 676
Re: delta u sign
Right - this is since the volume decreases (negative  V) and corresponds to a change from: - P
V) and corresponds to a change from: - P V to + P
V to + P V.
V.
- Fri Jan 13, 2017 4:04 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Bruincast
- Replies: 26
- Views: 10634
Re: Bruincast
Will any of the 14B lectures be Webcasted?
- Fri Jan 13, 2017 3:56 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed system
- Replies: 3
- Views: 851
Re: Closed system
I would assume the system is isolated because thermodynamic problems calculate changes in energy
- Fri Nov 25, 2016 8:07 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Discussion Section
- Replies: 1
- Views: 477
Re: Discussion Section
I think he mentioned something about going to another one of your TA's sections!
- Fri Nov 18, 2016 8:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Water in K calculation
- Replies: 3
- Views: 701
Re: Water in K calculation
If a product or reactant is a solid or liquid, is its contribution to the K calculation automatically 1?
- Mon Nov 14, 2016 12:40 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Sigma and Pi order in MO
- Replies: 1
- Views: 387
Re: Sigma and Pi order in MO
Correct me if I'm wrong but I think 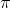 2p bond switches with the
2p bond switches with the 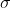 2p once z
2p once z 8 because there are actually electrons occupying the space for pi bonds.
8 because there are actually electrons occupying the space for pi bonds.
- Tue Nov 01, 2016 6:46 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Fall 2012 Midterm Q5B
- Replies: 2
- Views: 790
Fall 2012 Midterm Q5B
This questions asks for Lewis structures of a few gaseous molecules to see if they would contribute to global warming. Part (d) asks for SO2, which I drew S as the central atom double bonded with O on both sides - both O's and S have a lone pair in my drawing, which has zero formal charge on each at...
- Mon Oct 31, 2016 1:57 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Larger Molecular Shapes
- Replies: 1
- Views: 374
Larger Molecular Shapes
If a molecule has enough atoms to have different structures depending on which you count as the central atom, how would you describe the molecular shape? For example, (CH3)2Be is a linear structure when considering Be as the central atom, but the carbon would be considered tetrahedral on its own?
- Thu Oct 20, 2016 5:20 am
- Forum: Administrative Questions and Class Announcements
- Topic: About the "Rough Guideline"
- Replies: 4
- Views: 4554
Re: About the "Rough Guideline"
Generally, I base it on how far away the elements are from each other on the periodic table: the farther they are i.e. opposite sides of the periodic table, the more ionic in character.
- Wed Oct 19, 2016 10:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Workbook prep for Quiz #2
- Replies: 1
- Views: 454
Workbook prep for Quiz #2
I was looking at the practice exams in the workbook for Quiz #2 and saw that some of the questions were on things that aren't covered in Chapter 2 and 3 i.e. sigma and pi bonds, hybridization, bond order, etc. Do we still do these problems and turn them in next week when our discussion is?
- Sun Oct 16, 2016 11:28 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Posting on chem com
- Replies: 1
- Views: 461
Re: Posting on chem com
I'm under the assumption that the post dates coincide with the week count at the top righthand corner of my.ucla.edu (see attached photo)
- Sun Oct 09, 2016 8:54 pm
- Forum: Significant Figures
- Topic: All students read this sig fig post [ENDORSED]
- Replies: 170
- Views: 34747
Re: All students read this sig fig post [ENDORSED]
For testing purposes, is there a difference between writing 1.8 x 10^-2 and 0.018? I know they're synonymous, but I've seen both used in the textbook and workbook so I was wondering if there is a preference for either when it comes to giving a final answer.
- Fri Oct 07, 2016 3:48 pm
- Forum: Student Social/Study Group
- Topic: Hitch Study Group
- Replies: 12
- Views: 2177
Re: Hitch Study Group
Is anyone down to meet up Sunday night?
- Wed Sep 28, 2016 6:41 pm
- Forum: Student Social/Study Group
- Topic: Hitch Study Group
- Replies: 12
- Views: 2177
Re: Hitch Study Group
I'm in Hitch C, but would def wanna meet up and study sometime!

