An anti-Newman formation is when the two largest functional groups of each carbon are 180 degrees from each other. A gauche formation is when the two largest functional groups of each carbon are 60 degrees from each other.
Both these formation are variations of staggered.
Search found 12 matches
- Sun Mar 12, 2017 5:07 pm
- Forum: *Alkanes and Substituted Alkanes (Staggered, Eclipsed, Gauche, Anti, Newman Projections)
- Topic: High Energy
- Replies: 1
- Views: 501
- Sun Mar 12, 2017 5:01 pm
- Forum: *Ethers
- Topic: Ether Naming
- Replies: 3
- Views: 1939
Ether Naming
I'm still somewhat confused on the ether naming. Is it possible if someone can give and complete an example?
- Sun Feb 26, 2017 9:08 pm
- Forum: *Electrophiles
- Topic: Showing Electron Arrangement
- Replies: 1
- Views: 451
Showing Electron Arrangement
I noticed in the course reader on page 83 that there is a difference in the arrows used to show the movement of one electron and two electrons.
Is this important? On the quiz will we need to strictly use a one sided arrow to show only one electron moving?
Is this important? On the quiz will we need to strictly use a one sided arrow to show only one electron moving?
- Sun Feb 19, 2017 9:14 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: First order, second order reactions [ENDORSED]
- Replies: 6
- Views: 1434
Re: First order, second order reactions [ENDORSED]
We are tested on everything up to page 73 in the course reader. This page ends with "Pre-equilibrium approach".
EmilyLeibovitch2E wrote:Will we ever be asked to do a third or forth order reaction even though it wasn't covered in the course reader?
- Sun Feb 12, 2017 6:17 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: When to add H+ in cell diagram?
- Replies: 1
- Views: 561
When to add H+ in cell diagram?
On page 144 in the course reader, part C says to write the cell diagram and it includes H+ in the diagram.
How do we know when to use H+ in the diagram?
How do we know when to use H+ in the diagram?
- Fri Feb 03, 2017 7:13 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3649682
- Fri Feb 03, 2017 7:10 pm
- Forum: Balancing Redox Reactions
- Topic: How do I find oxidation number?
- Replies: 2
- Views: 699
Re: How do I find oxidation number?
Hi I had a similar question. My PLF gave me an answer with specific steps. 1. The oxidation state of a lone element or free state is zero. For example if you see a long element such as Cu, Zn, Ag. The O.S. will be zero. There are 8 diatomic elements to remember who's O.S. is zero as well: H2, O2, N2...
- Fri Jan 27, 2017 6:01 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Energy Extensive or Intensive?
- Replies: 2
- Views: 24517
Energy Extensive or Intensive?
I was looking at an old quiz and it asked if energy was extensive or intensive.
What is the difference between the two?
What is the difference between the two?
- Thu Jan 26, 2017 5:56 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Problem on pg 39
- Replies: 2
- Views: 501
Gibbs Free Energy Problem on pg 39
On page 39 in the course reader, there is an example problem Lavelle went over. It says that deltaG is equal to zero.
Why does the delta G equal to zero?
Why does the delta G equal to zero?
- Wed Jan 18, 2017 2:43 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Ch 8. Q.1 [ENDORSED]
- Replies: 2
- Views: 626
Re: Ch 8. Q.1 [ENDORSED]
Hi, I'm curious as to why d) gasoline burning in an automobile engine is an open system rather than closed? Since the engine is within the car, right? An open system is defined as a system where matter and energy can transfer with the surrounds. SO specifically with this question, because you are a...
- Wed Jan 18, 2017 2:37 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Work Integral Negative? [ENDORSED]
- Replies: 2
- Views: 582
Work Integral Negative? [ENDORSED]
Can someone explain why the formula in lecture for work is negative?
The formula was: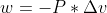
The formula was:
- Fri Jan 13, 2017 12:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies
- Replies: 7
- Views: 958
Re: Bond Enthalpies
In using this method, when a bond is formed do we write it as negative energy, and when a bond is broken, we write it as positive energy to add them up? When a bond a formed, energy is released so that will be positive energy. When the bond is broken, energy is used, making it negative. To find the...

