Search found 20 matches
Re: Naming
I think it can be both.
- Mon Mar 06, 2017 9:32 am
- Forum: *Cycloalkenes
- Topic: Naming Cycloalkenes
- Replies: 1
- Views: 662
Re: Naming Cycloalkenes
You would go with the one with more carbons, so you would use the chain as the base of the name and you would name the cycloalkane as a substituent. For example if we had a cyclobutane and a heptane chain attached, you would say cyclobutylheptane, since the heptane is the longer carbon chain.
- Wed Mar 01, 2017 1:05 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3659944
- Mon Feb 20, 2017 6:12 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Rate law of rxn mechanisms
- Replies: 1
- Views: 513
Re: Rate law of rxn mechanisms
According to the course reader, the pre-equilibrium approach is simpler, but less flexible. You also need the reaction before the rate limiting step is equilibrium. For the steady-state approach, the concentration of intermediate in the assumed to be constant.
- Mon Feb 13, 2017 1:18 pm
- Forum: Phase Changes & Related Calculations
- Topic: Equation to find standard reduction potentials?
- Replies: 2
- Views: 653
Re: Equation to find standard reduction potentials?
It is actually a table of values that they have found by experimentation when one is the standard hydrogen electrode.
- Mon Feb 06, 2017 1:37 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3659944
- Mon Jan 30, 2017 9:24 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies
- Replies: 3
- Views: 828
Re: Bond Enthalpies
Yes, you would multiply the bond enthalpies by the number of moles so that you can find the total amount required to break and form all the bonds.
- Mon Jan 23, 2017 6:21 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 8.31 HW problem
- Replies: 1
- Views: 499
Re: 8.31 HW problem
For part a, the molar heat capacity of a monatomic ideal gas at constant pressure, like Kr(g), is C P,m = (5/2)R. To calculate the heat that is released, we use q = nC P,m (T final -T initial ) to get -90.6J. We have all the information because we can convert the gas to the amount of moles of gas an...
- Thu Jan 19, 2017 11:54 am
- Forum: Phase Changes & Related Calculations
- Topic: Different enthalpy of vaporizations for water
- Replies: 3
- Views: 661
Re: Different enthalpy of vaporizations for water
The standard state just means what phase the substance is at 1atm and at 25 degrees Celsius. For example, the standard state of water under these conditions would be liquid. If it's not at 1atm, then it's not in its standard state, so you would find the reaction enthalpy.
- Thu Jan 12, 2017 9:17 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3659944
Re: Post All Chemistry Jokes Here
"I don't trust atoms...they make up everything."
- Thu Dec 01, 2016 1:28 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Homework Help 12.69
- Replies: 1
- Views: 538
Re: Homework Help 12.69
The Cl ion does not affect the pH of the solution at all so we can just ignore it. Only the NH4 affects the pH because it can donate a proton to H2O.
- Fri Nov 25, 2016 3:54 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Indicators of strong or weak acid/base
- Replies: 3
- Views: 852
Indicators of strong or weak acid/base
Hi, I am still sort of confused as to how we can tell if an acid or a base is strong or weak and why most inorganic acids are strong compared to organic acids. I know that strong acids lose a proton easily and the resulting anion must be stable, but I remember that we can somehow tell from K a and K...
- Tue Nov 15, 2016 6:05 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3659944
Re: Post All Chemistry Jokes Here
A man walks into a bar. He says, "I'll have some H20."
The man next to him says, "I'll have some H20 too!", then drinks it and dies.
The man next to him says, "I'll have some H20 too!", then drinks it and dies.
- Fri Nov 11, 2016 11:20 am
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: HW Problem 4.59.A
- Replies: 1
- Views: 488
Re: HW Problem 4.59.A
For all atoms with an atomic number less than 8 (so anything before oxygen), the pi orbital is more stable because it has less energy than the sigma orbitals for 2p, so they fill up first. For all atoms with an atomic number greater than or equal to 8, the sigma orbital fills up before the pi orbital.
- Thu Nov 03, 2016 11:13 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Bond order of B2 molecule
- Replies: 1
- Views: 52790
Re: Bond order of B2 molecule
The molecular orbital diagram for B 2 is http://img.sparknotes.com/figures/8/83ce1fb7be648ede5785ea60c96b495c/b2correlate.gif You can see that there are two electrons in the σ 2s and two electrons in the σ* 2s and two in the π 2p orbitals. We now know that there are two electrons in the antibonding ...
- Thu Oct 27, 2016 10:18 pm
- Forum: Sigma & Pi Bonds
- Topic: sigma/pi bonds [ENDORSED]
- Replies: 1
- Views: 598
Re: sigma/pi bonds [ENDORSED]
All of the single bonds are sigma bonds and for the double bond, one of the bonds is a pi bond.
For a single bond, there is a sigma bond.
For a double bond, there is a sigma bond and a pi bond.
For a triple bond, there is a sigma bond and two pi bonds.
For a single bond, there is a sigma bond.
For a double bond, there is a sigma bond and a pi bond.
For a triple bond, there is a sigma bond and two pi bonds.
- Tue Oct 18, 2016 12:46 am
- Forum: Trends in The Periodic Table
- Topic: Electron Affinity [ENDORSED]
- Replies: 3
- Views: 966
Re: Electron Affinity [ENDORSED]
Electron affinity is the amount of energy released when an electron is added to a gas-phase atom. A positive electron affinity means that energy is released when an electron is added. A negative electron affinity means that energy must be supplied for an atom to gain an electron.
- Thu Oct 13, 2016 8:07 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3659944
Re: Post All Chemistry Jokes Here
I'm a female.
Fe = Female
Male = Man
Therefore, I am Iron Man.
Fe = Female
Male = Man
Therefore, I am Iron Man.
- Thu Oct 06, 2016 4:28 pm
- Forum: Properties of Light
- Topic: Calculating Wavelength
- Replies: 1
- Views: 659
Re: Calculating Wavelength
We know that the frequency 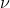 is 99.5 MHz, so you would be able to find the wavelength by using the equation
is 99.5 MHz, so you would be able to find the wavelength by using the equation 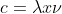 .
.
Substitute in the speed of light for c and your frequency above (remember to convert to Hz) (1 MHz = 10^6 Hz) to find the wavelength.
Substitute in the speed of light for c and your frequency above (remember to convert to Hz) (1 MHz = 10^6 Hz) to find the wavelength.
- Fri Sep 30, 2016 3:16 pm
- Forum: Properties of Light
- Topic: Increasing Intensity in Photoelectric Effect [ENDORSED]
- Replies: 9
- Views: 2287
Re: Increasing Intensity in Photoelectric Effect [ENDORSED]
Hi, from what I know, the threshold value is the minimum amount of energy it requires to remove an electron from the metal. It is also called the work function. And yes, the wavelength must be shorter to increase the frequency, which in turn increases the energy so that there is enough energy to rem...


