Search found 40 matches
- Fri Mar 17, 2017 11:16 am
- Forum: *Constitutional and Geometric Isomers (cis, Z and trans, E)
- Topic: Problem Set 2 Question
- Replies: 1
- Views: 1150
Problem Set 2 Question
Hello! I understand that when determining whether a molecule is cis or trans, we give the higher priority to the atom with the larger atomic molecule. In this case, I know Br has the higher priority, but how do we know that it is trans since on the other side, they are both CH3 and thus have the sam...
- Mon Mar 13, 2017 4:08 pm
- Forum: Balancing Redox Reactions
- Topic: 2013 Final #4
- Replies: 4
- Views: 959
2013 Final #4
Given  + 2H_{2}O(l) + O_{2} \rightarrow 2Fe(OH)_{2} (s)) , how would we write the half reactions? Thank you!
, how would we write the half reactions? Thank you!
- Sat Mar 11, 2017 3:42 pm
- Forum: *Ethers
- Topic: Homework Ch. 2 #30
- Replies: 3
- Views: 2701
Homework Ch. 2 #30
Could someone describe/break down their thought process in writing the IUPAC and the common name for 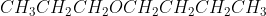 ? Thank you!
? Thank you!
- Mon Mar 06, 2017 12:19 pm
- Forum: *Alkanes
- Topic: Molecular Formula and Chemical Structure
- Replies: 2
- Views: 586
Molecular Formula and Chemical Structure
Hello! I know that alkanes have the general molecular formula: C_{n}H_{2n+2} and that any compound with this carbon and hydrogen ration must be an alkane. In the course reader, it asks if we are able to determine the structure from the molecular formula. It then says we do not know the structure fro...
- Fri Mar 03, 2017 12:32 am
- Forum: *Nucleophiles
- Topic: NH3+ as a nucleophile & Determining if Nucleo/Electrophile
- Replies: 2
- Views: 1357
NH3+ as a nucleophile & Determining if Nucleo/Electrophile
Why is NH3+ considered a nucleophile?
Additionally, what is the best way to determine if a molecule/atom is a nucleophile/electrophile? Is it best to just draw out the Lewis structures?
Thank you!
Additionally, what is the best way to determine if a molecule/atom is a nucleophile/electrophile? Is it best to just draw out the Lewis structures?
Thank you!
- Fri Feb 24, 2017 4:56 pm
- Forum: *Organic Reaction Mechanisms in General
- Topic: Determining Where Hydrogen Bonds
- Replies: 5
- Views: 1116
Determining Where Hydrogen Bonds
Hello! In the reaction mechanism example in the course reader, we said that the pi bond between the carbons in CH3CHCH3 was broken and that H--Br bond was broken. How did we know that the the carbon was partially positive and would be the atom to which the hydrogen would bond when a collision occurr...
- Sat Feb 18, 2017 2:26 pm
- Forum: Zero Order Reactions
- Topic: Zero Order and k [ENDORSED]
- Replies: 2
- Views: 768
Zero Order and k [ENDORSED]
Hello!
So I know that if we plot [A] vs time and we get a straight line, then the zero order reaction has a slope of -k. However, is it possible for k to be positive as well? Thank you!
So I know that if we plot [A] vs time and we get a straight line, then the zero order reaction has a slope of -k. However, is it possible for k to be positive as well? Thank you!
- Tue Feb 14, 2017 11:16 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Determine Cathode and Anode
- Replies: 2
- Views: 2052
Determine Cathode and Anode
Hello! I just wanted to make sure my reasoning is correct: If both of the half reactions are written as a reduction reactions, then the one with the higher reduction potential is more likely to be reduced, and is therefore the oxidizing agent and the cathode. The one with the lower reduction potenti...
- Sun Feb 12, 2017 5:18 pm
- Forum: Balancing Redox Reactions
- Topic: 2016 Midterm #8A
- Replies: 3
- Views: 634
2016 Midterm #8A
A standard electrochemical cell is made by placing a silver electrode into a 1.0 M Ag + solution and a cadmium electrode into a 1.0 M Cd2+ solution. What is the redox reaction and what is the maximum potential produced by this cell? Be sure to show half reactions? How do we tell which is being oxidi...
- Sat Feb 11, 2017 9:31 pm
- Forum: Balancing Redox Reactions
- Topic: Midterm 2015 #8C
- Replies: 1
- Views: 406
Midterm 2015 #8C
In 2003 researchers developed a microbial fuel cell. In this fuel cell, bacteria generates electricity as it aerobically converts glucose into carbon dioxide and water: The balanced chemical reaction of glucose oxidation. C_{6}H_{12}O_{6} (aq) + 6O_{2}(g) \rightarrow 6H_{2}O (l&#...
- Sat Feb 11, 2017 8:07 pm
- Forum: Balancing Redox Reactions
- Topic: 2014 Midterm #8 [ENDORSED]
- Replies: 3
- Views: 817
2014 Midterm #8 [ENDORSED]
Hello! The question is: Using the standard cell potentials: F_{2} (g)+ 2H^{+} (aq) + 2 e^{-}\rightarrow 2 HF (aq) Enot = +3.03V F_{2} (g) + 2 e^{-} \rightarrow 2 F^{-}(aq) Enot = +2.87V How do we determine which one is the cathode and which on is the anode bec...
- Fri Feb 10, 2017 9:46 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Different Forms of Nernst Equations
- Replies: 1
- Views: 1270
Different Forms of Nernst Equations
Hello! I know that there are basically two forms of the Nernst Equation that we can use: 1) E_{cell}=E^{\circ}_{cell}-\frac{RT}{nF}lnQ and 2) E_{cell}=E^{\circ}_{cell}-\frac{0.0592}{n}logQ . In which situations would we use 1) vs. 2)? Can we use them interchangeably, or we will we get different answ...
- Tue Feb 07, 2017 10:43 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Homework #11 Part D
- Replies: 1
- Views: 366
Homework #11 Part D
Write the half-reactions and the balanced equation for the cell reaction for each of the follow galvanic cells: Pt(s)\left | O_{2}(g) \right |H^{+}(aq)\left \| OH^{-}(aq)\left | O_{2} (g)\right |Pt(s) How would you approach this problem, given that the...
- Tue Jan 31, 2017 10:34 pm
- Forum: Calculating Work of Expansion
- Topic: When to Use Different Formulas for Work
- Replies: 2
- Views: 602
When to Use Different Formulas for Work
In which situations/under which conditions would we use  or
or  ?
?
- Mon Jan 30, 2017 1:11 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Winter 2013 Midterm #5.B
- Replies: 1
- Views: 490
Winter 2013 Midterm #5.B
Arrange the following substances in order of increasing standard molar entropy" CHF_{3}(g), CF_{4}(g), CH_{3}F(g), CH_{2}F_{2} (g) . The answer is given as: CH_{3}F(g) < CH_{2}F_{2} (g) < CHF_{3}(g) < CF_{4}(g) . I know that standa...
- Mon Jan 30, 2017 1:07 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Relationship between Standard Molar Entropy and Molecular Complexity (9.71)
- Replies: 2
- Views: 683
Re: Relationship between Standard Molar Entropy and Molecular Complexity (9.71)
Comparing the molecular structures, you can see that benzene is unique for having double bonds between the carbons (sp 2 hybridized), so for each carbon there are 3 of sp2 hybrized orbitals interacting with the surrounding 2 carbons and 1 hydrogen. Since sp 2 hybridized orbitals are bonding, that l...
- Sat Jan 28, 2017 9:25 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Quiz #1 Preparation #12
- Replies: 1
- Views: 500
Re: Quiz #1 Preparation #12
Yes, you would need to use \Delta G = \Delta H -T\Delta S. Since you know that \Delta H_{rxn}= \Sigma \Delta H_{f}(products)-\Sigma \Delta H_{f}(reactants) and \Delta S_{rxn}= \Sigma \Delta S(products)-\Sigma \Delta S(reactants) , then we can use \Delta G = \Delta H -...
- Wed Jan 25, 2017 10:47 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Relationship between Standard Molar Entropy and Molecular Complexity (9.71)
- Replies: 2
- Views: 683
Relationship between Standard Molar Entropy and Molecular Complexity (9.71)
In the Course Reader, on p. 36, it says that "More complex molecules have higher S^{\circ} due to a greater number of vibrational and rotational motions (atoms have a greater number of possible positions" which I understand. However,one of the homework questions, 9.71 asked "Which sub...
- Wed Jan 18, 2017 5:55 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: HW 8.13 & Heat Absorption
- Replies: 2
- Views: 519
HW 8.13 & Heat Absorption
"In a combustion cylinder, the total internal energy changes produced from the burning of a fuel is -2573 kJ. The cooling system that surrounds the cylinder absorb 947 kJ as heat. How much work can be done by the fuel in the cylinder?" I know to use \Delta U = q + w. So \Delta U = -2573 kJ...
- Tue Jan 17, 2017 9:49 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Using Bond Enthalpies to Calculate Total Enthalpy Change [ENDORSED]
- Replies: 2
- Views: 623
Using Bond Enthalpies to Calculate Total Enthalpy Change [ENDORSED]
When we use bond enthalpies to calculate the total enthalpy change, how can we tell which bonds are broken and which bonds are formed? Is this skill more intuitive or is there a specific way to tell which bonds are broken/formed i.e looking at Lewis structures or the chemical reaction? Thank you!
- Thu Jan 12, 2017 11:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpy and Standard State
- Replies: 2
- Views: 489
Standard Reaction Enthalpy and Standard State
I know that when reactants and products are in their standard state at 1 atm, the reaction enthalpy is the standard reaction enthalpy. However, how do we know that a reactant/product is in its standard state for any element? For example, I know that N_{2} is the standard state for N, but why is that...
- Wed Nov 30, 2016 11:15 pm
- Forum: Bronsted Acids & Bases
- Topic: 12.9 part d
- Replies: 1
- Views: 521
12.9 part d
Which of the following reactions can be classified as reactions between Bronsted acids and bases? For those that can be so classified, identify the acid and base. (Hint: It may help to write the net ionic equations.) d) NH_{4}I (am) + KNH_{2}(am) \rightarrow KI(am) + 2 NH_{3}...
- Tue Nov 29, 2016 11:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 11.117
- Replies: 2
- Views: 914
11.117
Hi! I know somebody already asked this question, but I was still confused about the explanation. The question reads: "The two air pollutants SO3 and NO can react in the atmosphere as follows: SO_{3} (g) + NO(g)\rightarrow SO_{2}(g) + NO_{2}(g) . b) Give that a t ...
- Tue Nov 29, 2016 4:56 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Ions as Bases in CR
- Replies: 1
- Views: 527
Ions as Bases in CR
In the Course Reader, on page 160, it says that a salt containing a base will raise solution pH. When it says solution, is it just referring to a solution of the salt and water? How exactly does the salt increase the concentration of OH-? Also, it says that salts that contain the conjugate base (ani...
- Tue Nov 29, 2016 1:28 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Approximating X
- Replies: 2
- Views: 713
Approximating X
When trying to calculate the equilibrium compositions, how can we check to make sure that we can approximate x (by removing it from the denominator of the equilibrium constant calculation) and that is valid? I think Dr. Lavelle said something about it being valid if x was less than 5% of the initial...
- Mon Nov 28, 2016 11:32 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Response of Equilibria to Change in CR pg. 138
- Replies: 2
- Views: 485
- Mon Nov 28, 2016 11:18 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Response of Equilibria to Change in CR pg. 138
- Replies: 2
- Views: 485
Response of Equilibria to Change in CR pg. 138
On page 138 in the Course Reader, we are given the example  + 3H_{2}(g) \leftrightharpoons 2NH_{3}(g)) . Could you please explain what happens if H2 is decreased and why? Thank you!
. Could you please explain what happens if H2 is decreased and why? Thank you!
- Tue Nov 22, 2016 8:50 pm
- Forum: Conjugate Acids & Bases
- Topic: Textbook Problem 12.37
- Replies: 1
- Views: 1694
Textbook Problem 12.37
For each of the following weak acids, write the proton transfer equilibrium equation and the expression for the equilibrium constant K_{a} . Identify the conjugate base, writ ether appropriate proton transfer equation, and write the expression for basicity constant K_{b} .(a) HClO_{2} (b) HCN (c) C_...
- Fri Nov 18, 2016 12:34 am
- Forum: Bronsted Acids & Bases
- Topic: Strong and Weak Acids
- Replies: 1
- Views: 549
Strong and Weak Acids
Hello!
How would we determine if an acid is strong or weak? I understand that strong acids produce more proton in a solution than a weak acid, but how would we know that? Thank you!
How would we determine if an acid is strong or weak? I understand that strong acids produce more proton in a solution than a weak acid, but how would we know that? Thank you!
- Fri Nov 11, 2016 10:01 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: FALL 2015 #4 [ENDORSED]
- Replies: 1
- Views: 385
FALL 2015 #4 [ENDORSED]
For the complex ions listed below, determine the oxidation state and the coordination number of the central metal ion? [Fe(Cl)_{4}(en)]^{-2^} I know that the oxidation state is +2, but I am confused on how to get the coordination number. I know that the number of bonds is equal to th...
- Tue Nov 08, 2016 12:10 am
- Forum: Hybridization
- Topic: Problem 4.91
- Replies: 1
- Views: 1207
Problem 4.91
Hello! The question says: Benzyne, C6H4, is a highly reactive molecule that is detected only at low temperatures. It is related to benzene in that it has a six-membered ring of carbon atoms; but, instead of three double bounds, the structure is normally drawn with two double bonds and a triple bond....
- Tue Nov 01, 2016 10:36 am
- Forum: Hybridization
- Topic: Regions of Electron Density & Naming Hybrid Orbitals
- Replies: 3
- Views: 715
Regions of Electron Density & Naming Hybrid Orbitals
Hello! My understanding of how to find the type of hybrid orbitals is to just count the regions of the electron densities and ensure that I have the corresponding hybrid orbital. For example, if there were to be three regions of electron density around the central atom, that means that the central a...
- Fri Oct 28, 2016 12:27 am
- Forum: Hybridization
- Topic: Question About Methane Structure and Carbon Bonding
- Replies: 2
- Views: 1482
Question About Methane Structure and Carbon Bonding
Hello! In the course reader on page 102, it says that that methane (CH4) structure has 4 equivalent covalent bonds that point towards the four corners of tetrahedron. It then says that carbon has only two unpaired electrons. Can someone explain that if C has only 2 unpaired electrons, how does it fo...
- Fri Oct 21, 2016 5:26 pm
- Forum: Resonance Structures
- Topic: Homework Problem 3.95
- Replies: 2
- Views: 591
Homework Problem 3.95
Hello! The question reads "Draw resonance structures for the trimethylenemethane anion C(CH2)32- in which a central carbon atom is attached to three CH2 groups (CH2 groups are referred to as methylene).
Thank you!
Thank you!
- Wed Oct 19, 2016 2:25 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Problem 2.47
- Replies: 1
- Views: 478
Problem 2.47
For each of the following ground-state atoms, predict the type of orbital from which an electron will be removed to form the +1 ion. (a) Ge; (b) Mn; (c) Ba, and (d) Au. Can someone please explain how they are going about this question? I'm getting the correct answers, but would like to know if I'm d...
- Fri Oct 14, 2016 2:24 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Problem 2.17
- Replies: 2
- Views: 409
Problem 2.17
"How many orbitals are in subshells with l equal to (a) 0; (b) 2; (c) 1; (d) 3?"
I know that this is a fairly basic question, but could some please explain how they got to their answer in detail and the reasoning behind the answer? Thank you!
I know that this is a fairly basic question, but could some please explain how they got to their answer in detail and the reasoning behind the answer? Thank you!
- Thu Oct 13, 2016 6:51 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Audio Visual Focus Topics, Assessments, and Surveys for Ch. 2
- Replies: 1
- Views: 370
Audio Visual Focus Topics, Assessments, and Surveys for Ch. 2
Is it possible that video modules and assessments on atomic orbitals/wave function electron configurations could be created and/or posted? I personally found the video modules and assessments to be extremely helpful when reviewing and checking my understanding of the material lectured on in the clas...
- Sat Oct 08, 2016 3:03 pm
- Forum: Limiting Reactant Calculations
- Topic: Fundamentals M.9 [ENDORSED]
- Replies: 3
- Views: 917
Fundamentals M.9 [ENDORSED]
Copper (II) nitrate reacts with sodium hydroxide to produce a precipitate of light blue copper(II) hydroxide. (a) Write the net ionic equation for the reaction. I am a bit rusty on this, so my question is, how would we go about writing the net ionic equation for this particular reaction, or any reac...
- Thu Oct 06, 2016 5:50 pm
- Forum: Photoelectric Effect
- Topic: Given the kinetic energy, how do you find the energy? [ENDORSED]
- Replies: 4
- Views: 1695
Re: Given the kinetic energy, how do you find the energy? [ENDORSED]
The difference between the kinetic energy and the energy of the incident light is that the kinetic energy refers to the [b]energy of the ejected electron[/b ]. This can be found using the equation E_{k}=0.5\cdot m\cdot v^{2} , where m = mass of the electron and v = velocity of the ejected electron. ...
- Thu Sep 29, 2016 9:45 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Post-Module Assessment #6
- Replies: 1
- Views: 417
Post-Module Assessment #6
#6 in the Post-Module Assessment asks "In an atomic absorption spectrum what does one line (one wavelength) represent?" I understand the concept that atoms can only absorb and emit certain energies and that a line corresponds to the transition between two of the "allowed" energy ...

