Relation between k and activation energy
Moderators: Chem_Mod, Chem_Admin
-
Mishta Stanislaus 1H
- Posts: 45
- Joined: Fri Sep 29, 2017 7:04 am
Relation between k and activation energy
What exactly is the relationship between k and activation energy?
-
Lily Sperling 1E
- Posts: 49
- Joined: Tue Oct 10, 2017 7:14 am
Re: Relation between k and activation energy
You can observe the relationship through the Arrhenius Equation - basically, the formula implies that the rate constant increases exponentially as the activation energy decreases.
-
Christina Cen 2J
- Posts: 53
- Joined: Sat Jul 22, 2017 3:01 am
Re: Relation between k and activation energy
Reactions generally speed up when temperature increases and slow down when temperature decreases. This means that k increases with increasing temperature, which in turn increases the overall rate of the reaction.
-
diangelosoriano
- Posts: 49
- Joined: Fri Sep 29, 2017 7:07 am
Re: Relation between k and activation energy
If a catalyst were added, how would this also affect k or activation energy?
-
Johann Park 2B
- Posts: 51
- Joined: Thu Jul 27, 2017 3:01 am
- Been upvoted: 1 time
Re: Relation between k and activation energy
diangelosoriano wrote:If a catalyst were added, how would this also affect k or activation energy?
When you add a catalyst, the reaction is sped up - k is increased and Ea is lowered. The new pathway has a lower activation energy.
-
Nehal Banik
- Posts: 64
- Joined: Thu Jul 13, 2017 3:00 am
Re: Relation between k and activation energy
As activation energy decreases, it reduces the energy barrier required for the reaction to proceed therefore the rate constant increases, causes the overall rate of the reaction to increase. Also, if the activation energy is higher, the dependence of the rate constant on temperature increases, meaning at higher temperatures the rate constant is most likely going to be higher. These are a couple of the relationships that can be understood from the Arrhenius equation in the textbook.
Hope that helps!
Hope that helps!
Re: Relation between k and activation energy
Anyone have a video they think is a good reference for this topic?
-
Joshua Eidam 2A
- Posts: 89
- Joined: Wed Sep 30, 2020 9:58 pm
Re: Relation between k and activation energy
diangelosoriano wrote:If a catalyst were added, how would this also affect k or activation energy?
Adding a catalyst is going to decrease the activation energy, Ea, which will ultimately lead to an increase in the rate constant k.
-
Joshua Eidam 2A
- Posts: 89
- Joined: Wed Sep 30, 2020 9:58 pm
Re: Relation between k and activation energy
A clear way to look at the relationship between k and the activation energy, Ea, is through the Arrhenius equation, 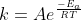 . This equation shows how a decrease in activation energy would ultimately lead to an increase in k and vice versa. Hope this helps!
. This equation shows how a decrease in activation energy would ultimately lead to an increase in k and vice versa. Hope this helps!
Re: Relation between k and activation energy
Using the equation k= Ae^-Ea/RT, you can determine that adding a catalyst will decrease the activation energy (Ea); with a decrease of Ea comes an increase in k, the rate constant.
-
Kathryn Heinemeier 3H
- Posts: 104
- Joined: Fri Sep 24, 2021 5:09 am
Re: Relation between k and activation energy
When a catalyst is added, the activation energy will decrease while the rate constant, k will increase.
Return to “*Free Energy of Activation vs Activation Energy”
Who is online
Users browsing this forum: No registered users and 2 guests

