Temperature vs. activation energy
Moderators: Chem_Mod, Chem_Admin
Temperature vs. activation energy
How does temperature affect rate constant k? Does an increase in temperature cause an increase in the rate constant?
-
Vincent Leong 2B
- Posts: 207
- Joined: Fri Aug 09, 2019 12:15 am
Re: Temperature vs. activation energy
Yes. If you increase T = more collisions = increased rxn rate (knowing rate = k[A]) = increased k
-
Indy Bui 1l
- Posts: 99
- Joined: Sat Sep 07, 2019 12:19 am
Re: Temperature vs. activation energy
Just because we are increasing the number of collisions, how does this correlate to a decrease in activation energy? I thought that is something a catalyst does.
-
Ayushi2011
- Posts: 101
- Joined: Wed Feb 27, 2019 12:17 am
-
Charlene Datu 2E
- Posts: 62
- Joined: Wed Sep 11, 2019 12:16 am
Re: Temperature vs. activation energy
As the temperature increases, the amount of energy in the system increases. This energy is then used to overcome the activation energy
-
Susan Chamling 1F
- Posts: 101
- Joined: Wed Sep 30, 2020 9:42 pm
Re: Temperature vs. activation energy
Yes, an increase in temperature means an increase in the energy within the system, which in turn increases the reaction rate.
-
alebenavides
- Posts: 118
- Joined: Wed Sep 30, 2020 9:39 pm
-
AnjikaFriedman-Jha2D
- Posts: 101
- Joined: Wed Sep 30, 2020 9:55 pm
Re: Temperature vs. activation energy
As the temperature increases, the energy of the system also increases
-
Gerardo Ortega 2F
- Posts: 111
- Joined: Wed Sep 30, 2020 9:59 pm
- Been upvoted: 1 time
Re: Temperature vs. activation energy
Yes, an increase in temperature causes an increase in the rate constant.
-
Katie Phan 1K
- Posts: 104
- Joined: Wed Sep 30, 2020 9:55 pm
-
AnjikaFriedman-Jha2D
- Posts: 101
- Joined: Wed Sep 30, 2020 9:55 pm
-
Bryan Le 2K
- Posts: 59
- Joined: Thu Dec 17, 2020 12:20 am
Re: Temperature vs. activation energy
When you increase the temperature, this results in more collisions between particles. This will lead to an increase in K.
-
Sahaj Patel Lec3DisK
- Posts: 130
- Joined: Wed Sep 30, 2020 10:03 pm
- Been upvoted: 1 time
Re: Temperature vs. activation energy
Temperature affects K depending on the type of reaction. If the reaction is exothermic, the more temperature increases the lower K will get because the production of reactants are favored. If the reaction is endothermic, the more temperature increases the higher K will get because the formation of products are favored. Hope this helps!
-
Moura Girgis 1F
- Posts: 109
- Joined: Wed Sep 30, 2020 10:00 pm
Re: Temperature vs. activation energy
As T increases, so too does K. As a result, increased T leads to more activation energy as a result of more collisions.
-
Katelynn Shaheen 2C
- Posts: 51
- Joined: Tue Nov 05, 2019 12:18 am
Re: Temperature vs. activation energy
An increase in temperature (T) causes more collisions. This increases the reaction rate which is why k increases when the temperature does.
-
Dylan_K_3B
- Posts: 50
- Joined: Wed Nov 18, 2020 12:24 am
- Been upvoted: 1 time
Re: Temperature vs. activation energy
On the equation sheet, there is also the equation: k = A * exp(-Ea/RT). If you examine this reaction, you can rewrite it as k = A * 1/exp(Ea/RT). The bigger T becomes, the smaller exp(Ea/RT) becomes, and the smaller the denominator of the fraction becomes, the bigger 1/exp(Ea/RT) becomes, leading to a larger k value.
-
Muskaan Abdul-Sattar
- Posts: 101
- Joined: Wed Nov 13, 2019 12:19 am
Re: Temperature vs. activation energy
Yes an increase in temperature will lead to an increase in K because it causes an increase in collisions.
-
Kyla Roche 2K
- Posts: 104
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Temperature vs. activation energy
If temperature increases, there are more collisions, thus the rate constant increases.
-
Colin Squire 3B
- Posts: 100
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Temperature vs. activation energy
If I remember correctly, yes, the relationship is proportional. An increase in T will result in an increase in K.
-
Lorena_Morales_1K
- Posts: 53
- Joined: Wed Sep 30, 2020 10:11 pm
Re: Temperature vs. activation energy
The relationship between temperature and the reaction rate is proportional; if you increase the temperature, you increase the reaction rate
-
Joshua Eidam 2A
- Posts: 89
- Joined: Wed Sep 30, 2020 9:58 pm
Re: Temperature vs. activation energy
Yes! If you look at the Arrhenius equation, 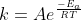 , you can clearly see how temperature affects the rate constant. If the temperature of the reaction is higher then the power to which e is multiplied will be a smaller negative number (aka more positive), and therefore the value of k will increase. Hope this helps.
, you can clearly see how temperature affects the rate constant. If the temperature of the reaction is higher then the power to which e is multiplied will be a smaller negative number (aka more positive), and therefore the value of k will increase. Hope this helps.
-
Kelly Tran 1J
- Posts: 152
- Joined: Wed Sep 30, 2020 9:50 pm
- Been upvoted: 1 time
Re: Temperature vs. activation energy
As others have stated above, an increase in temperature increases the rate constant, k. Similarly, a decrease in temperature decreases k.
-
Vincent Real 2C
- Posts: 43
- Joined: Mon Jan 03, 2022 8:38 pm
Re: Temperature vs. activation energy
An increase in temperature increases the rate constant K. This is because when temperature increases, there are more collisions in the given chemical reaction. As a result, products are formed faster, meaning the concentration increases. Since this is the numerator in a rate constant, this means that the rate constant increases.
-
Kathryn Heinemeier 3H
- Posts: 104
- Joined: Fri Sep 24, 2021 5:09 am
Re: Temperature vs. activation energy
Increase in temperature leads to an increase in energy in the system and it also helps create more products from the reaction which affects and increases k
-
Tara Cumiskey 3K
- Posts: 106
- Joined: Fri Sep 24, 2021 5:11 am
-
raynebunado
- Posts: 101
- Joined: Fri Sep 24, 2021 6:58 am
Re: Temperature vs. activation energy
temperature and reaction rate have a direct relationship; an increase in temperature results in an increase in reaction constant k
-
Amy Jordan 2A
- Posts: 104
- Joined: Fri Sep 24, 2021 6:23 am
Re: Temperature vs. activation energy
Hi, when temperature increases there is a corresponding increase in energy which causes k to increase as well.
-
Esther Kim
- Posts: 99
- Joined: Fri Sep 24, 2021 6:07 am
Re: Temperature vs. activation energy
Increase in T will increase K because the atoms will be more likely to collide
-
Jacob_Eberson_2D
- Posts: 50
- Joined: Mon Jan 03, 2022 8:40 pm
Re: Temperature vs. activation energy
A greater temperature means more collisions and an increased rate of reaction.
Re: Temperature vs. activation energy
Increasing the temperature causes the molecules to react with more kinetic energy. This increases the probability of collisions between molecules. Therefore, this increases the rate of the reaction because there are more molecules with enough kinetic energy to pass the activation energy barrier.
-
Kavya Anand 2B
- Posts: 101
- Joined: Fri Sep 24, 2021 5:25 am
Re: Temperature vs. activation energy
Increasing the temperature does cause an increase in k, as increasing the temperature increases the energy in the reaction, therefore causing an increased amount of collisions that speed up the reaction.
-
Vanessa Bartoli 1C
- Posts: 52
- Joined: Mon Jan 03, 2022 10:34 am
Return to “*Free Energy of Activation vs Activation Energy”
Who is online
Users browsing this forum: No registered users and 8 guests

