Difference between bond angle strain and steric strain
Moderators: Chem_Mod, Chem_Admin
-
shannon_tseng_3L
- Posts: 20
- Joined: Wed Sep 21, 2016 2:56 pm
Difference between bond angle strain and steric strain
What's the difference between bond angle strain and steric strain?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Difference between bond angle strain and steric strain
Great question. The two can be related, but not always. The simple answer is that steric strain is strain that cannot be alleviated by just changing the bond angle.
For example, cyclopropane and cyclobutane both exhibit high angular strain, but not really any steric strain. The best example of steric strain is that which we studied in class, namely the various conformations of butane. When the two end carbons are "eclipsed" over each other, the steric strain is much higher than if the end carbons (think of them as methyl groups) are staggered or "gauche." The image below demonstrates this.
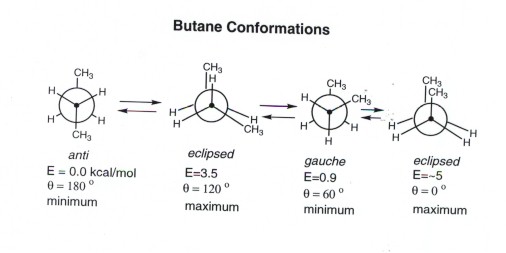
Image credit: http://research.cm.utexas.edu
For example, cyclopropane and cyclobutane both exhibit high angular strain, but not really any steric strain. The best example of steric strain is that which we studied in class, namely the various conformations of butane. When the two end carbons are "eclipsed" over each other, the steric strain is much higher than if the end carbons (think of them as methyl groups) are staggered or "gauche." The image below demonstrates this.

Image credit: http://research.cm.utexas.edu
-
AnnaTong1E
- Posts: 20
- Joined: Fri Jul 15, 2016 3:00 am
Re: Difference between bond angle strain and steric strain
Is there a clear definition for steric strain?
Return to “*Cyclopropanes and Cyclobutanes”
Who is online
Users browsing this forum: No registered users and 1 guest

