Im a bit confused on how to use different methods to solve for empirical and molecular formulas...
Is it necessary to find the percent composition in order to find the empirical formula? For example, the question involving 339.20 g of cobalt and fluorine gas to produce 996.08 g of a product, and i'm trying to find the empirical formula...would I have to first find the percent composition even though I know the mass of both the reactants and the final product?
Solving For Empirical and Molecular Formulas
Moderators: Chem_Mod, Chem_Admin
-
josephyim1L
- Posts: 61
- Joined: Fri Sep 28, 2018 12:15 am
-
Brandon_Tran_2E
- Posts: 63
- Joined: Fri Sep 28, 2018 12:23 am
Re: Solving For Empirical and Molecular Formulas
Yes it is necessary to find the mass % in order to solve the question unless it is given to you. For that particular question you would have to subtract 339.2 grams of Cobalt from the 996.08 grams of the compound to get 656.88 grams of the Fluorine gas.
Then you would divide the Cobalt and Fluorine gas by the total compound to get percent.
(339.20 g Co)/(996.08 g total)= 34.05 % Cobalt. (656.88 g F)/(996.08 g total)= 65.95 % Fluorine.
Assume 100 grams for the sample and use the mass percentages to solve for the moles.
(34.05 g Co)/(58.93 g/mol Co)= 0.5778 mol Co. And, (65.95 g F)/(19 g/mol F)= 3.473 mol F.
Next, divide all elements by the smallest amount of moles, (Cobalt) Ratio would be 1 cobalt for 6 fluorine
The empirical formula would then be CoF6.
Then you would divide the Cobalt and Fluorine gas by the total compound to get percent.
(339.20 g Co)/(996.08 g total)= 34.05 % Cobalt. (656.88 g F)/(996.08 g total)= 65.95 % Fluorine.
Assume 100 grams for the sample and use the mass percentages to solve for the moles.
(34.05 g Co)/(58.93 g/mol Co)= 0.5778 mol Co. And, (65.95 g F)/(19 g/mol F)= 3.473 mol F.
Next, divide all elements by the smallest amount of moles, (Cobalt) Ratio would be 1 cobalt for 6 fluorine
The empirical formula would then be CoF6.
-
bonnie_schmitz_1F
- Posts: 81
- Joined: Fri Sep 28, 2018 12:24 am
- Been upvoted: 1 time
Re: Solving For Empirical and Molecular Formulas
In this case, I'm not sure its completely necessary to find the percent composition because the question you're referring did not ask for it. Using the percent composition is definitely one way to do the problem, but you could do it another way since you know the mass of both substances.
Here is my work on the problem to help answer your question!
339.20 g Co * (1 mol Co/38.93 g Co) = 5.756 mol Co
656.88 g F * (1 mol F/19.00 g F) = 34.57 mol F
Then divide by the smallest number of moles
5.756/5.756 = 1 Co
34.57/5.756 = 6 F
So the result of the empirical formula is the same!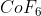
Here is my work on the problem to help answer your question!
339.20 g Co * (1 mol Co/38.93 g Co) = 5.756 mol Co
656.88 g F * (1 mol F/19.00 g F) = 34.57 mol F
Then divide by the smallest number of moles
5.756/5.756 = 1 Co
34.57/5.756 = 6 F
So the result of the empirical formula is the same!
-
Saachi_Kotia_4E
- Posts: 68
- Joined: Fri Sep 28, 2018 12:23 am
Re: Solving For Empirical and Molecular Formulas
If you first find the mass %, you can then easily assume that there is 100g of sample in order to determine the empirical formula. Then, you can use the mass percentages to find the number of moles of Co and F. After that, divide both values by the smaller of the two values in order to determine the ratio of each atom in the empirical formula. Finally, you can then use the total mass given in the problem and divide it by the mass of the empirical formula (calculated by adding up the molecular masses found in the periodic table). This number will let you know what to multiply the empirical formula by in order to determine the molecular formula.
-
AngelaZ 1J
- Posts: 65
- Joined: Fri Sep 28, 2018 12:23 am
Re: Solving For Empirical and Molecular Formulas
Finding the mass percentage would not be needed for this question. Unless the question asks for the mass percentages, I wouldn't take an extra step to find it when the moles can be calculated directly from the masses given. However, it doesn't hurt to calculate the mass percentage, so it can be up to a matter of preference.
Return to “Empirical & Molecular Formulas”
Who is online
Users browsing this forum: No registered users and 5 guests

