homework problem E9
Moderators: Chem_Mod, Chem_Admin
-
emma brinton_3B
- Posts: 53
- Joined: Fri Aug 09, 2019 12:17 am
homework problem E9
I was attempting to do this problem and saw in the solutions manual that you have to multiply the MgSO4 by 7 H2O to get the formula mass of 246.48 g x mol^-1. Does anyone understand why this step has to be done in order to get the answer?
-
RyanKopeikin_2I
- Posts: 81
- Joined: Fri Aug 30, 2019 12:17 am
Re: homework problem E9
Basically MgSO4 X 7H20 is the chemical equation for magnesium sulfate heptahydrate. As you can see, "hepta" is the prefix for 7, and hydrate means H20. So in order to calculate the molar mass of magnesium sulfate heptahydrate, you need to write out the full chemical equation so you can make sure to add up the molar masses of each atom to get the total. You do not actually multiply by 7H20. So really Mg +S+4(O)+14(H)+7(O)=246.48 g/mol.
-
Lauren Tanaka 1A
- Posts: 109
- Joined: Sat Aug 17, 2019 12:18 am
Re: homework problem E9
Since the problem says that epsom salts consist of magnesium sulfate heptahydrate, you have to multiply MgSO4 by 7H20. MgSO4 is the magnesium sulfate and 7H20 is the heptahydrate. After finding the formula for the compound you then have to multiply the given mass of the compound by the molar mass of the compound (246.48 g/mol). This will leave you with the amount of moles of MgSO47H2O (magnesium sulfate heptahydrate). Hope this helps!
-
Abigail Menchaca_1H
- Posts: 104
- Joined: Sat Sep 07, 2019 12:19 am
Re: homework problem E9
The question is saying that epsom salts contain magnesium sulfate (MgSO4) heptahydrate (7H2O). Essentially, you're just writing the magnesium sulfate heptahydrate formula out.
Last edited by Abigail Menchaca_1H on Tue Oct 01, 2019 10:51 pm, edited 1 time in total.
-
Ryan Narisma 4G
- Posts: 104
- Joined: Fri Aug 30, 2019 12:18 am
Re: homework problem E9
Hi there! So the compound at question is called Magnesium Sulfate Heptahydrate (MgSO

7H O). This specific salt is made of Magnesium (Mg
O). This specific salt is made of Magnesium (Mg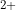 ), Sulfate (SO
), Sulfate (SO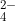 ), and water (H
), and water (H O) in a 1:1:7 ratio. In other words, to make Magnesium Sulfate Heptahydrate it must include 7 moles of water per 1 mole of Magnesium ion and 1 mole of Sulfate ion. This type of salt is a "hydrate" which contains water molecules in its crystalline structure. So, it's a solid with water molecules in the molecular structure. There are salts that do not contain water in the crystal and are therefore named "anhydrous". I hope this helps!
O) in a 1:1:7 ratio. In other words, to make Magnesium Sulfate Heptahydrate it must include 7 moles of water per 1 mole of Magnesium ion and 1 mole of Sulfate ion. This type of salt is a "hydrate" which contains water molecules in its crystalline structure. So, it's a solid with water molecules in the molecular structure. There are salts that do not contain water in the crystal and are therefore named "anhydrous". I hope this helps!
7H
-
emma brinton_3B
- Posts: 53
- Joined: Fri Aug 09, 2019 12:17 am
Re: homework problem E9
thanks so much for the help! does anyone by chance understand how to solve part B of this problem?? which is asking how many formula units of the compound are present in 5.15 g of epsom salt?
-
faithkim1L
- Posts: 105
- Joined: Fri Aug 09, 2019 12:17 am
Re: homework problem E9
The molar mass (MM) is the mass of the whole molecule, which includes the heptahydrate. Hepta means 7, so you need to multiply 18.02 g by 7 in order to get the mass of the heptahydrate. After that, you can just add the MM of the molecule MgSO4 to find the total molar mass of the molecule.
Return to “Empirical & Molecular Formulas”
Who is online
Users browsing this forum: No registered users and 2 guests

