Fundamental F question 7
Moderators: Chem_Mod, Chem_Admin
Fundamental F question 7
I’m having trouble finding the molar mass of the unknown metal, I set the mass of the compound to 100g but I do not know how I’d get to the molar mass of the metal. How would you get to the molar mass with only the mass percentage of it?
-
Anish Patel 4B
- Posts: 59
- Joined: Thu Jul 11, 2019 12:17 am
Re: Fundamental F question 7
The question gives you the formula for the desired compound as M2O. This means that there are twice as many moles of oxygen as there are for M. So, we can determine the mass of O in the compound by first assuming the mass of the compound to be 100g. As a result, 100-88.8 = 11.2 grams of oxygen. We convert this to moles by dividing the amount by the molar mass of O, 11.2g/16.00g = 0.700 mol O. Multiplying this by two gives us 1.40 mol of M in the compound. Through dimensional analysis, we can use the 88.8g of M and divide it by the 1.40 moles to find the molar mass, which ends up being 63.4 g/mol.
Re: Fundamental F question 7
From the formula there are 2 moles of M to every mole of O.
The relative number of moles of O = 11.2 % /16 (Molar mass oxygen)
The relative number of moles of M = 88.8% / Molar mass of M
But you know that 88.9/Molar mass M = 2 x 11.2/16
rearrange to give Molar mass M = 88.9/ (2 x 11.2 / 16) = 88.9 x 16 / 22.4 = 63.5
M is Copper and the Compound is Copper (I) Oxide Cu2O
The relative number of moles of O = 11.2 % /16 (Molar mass oxygen)
The relative number of moles of M = 88.8% / Molar mass of M
But you know that 88.9/Molar mass M = 2 x 11.2/16
rearrange to give Molar mass M = 88.9/ (2 x 11.2 / 16) = 88.9 x 16 / 22.4 = 63.5
M is Copper and the Compound is Copper (I) Oxide Cu2O
-
Julie Park 1G
- Posts: 100
- Joined: Thu Jul 25, 2019 12:15 am
Re: Fundamental F question 7
If you set the mass of the compound at 100g and you know that 88.8% of the compound is M (Metal), you can conclude that the metal weighs 88.8 grams (100g x 0.888). If the compound weighs 100g in total, then subtracting the amount of metal from it should give you the weight of the leftover component, which in this case, is oxygen (O). Since 100g-88.8g is 11.2g, you therefore have 11.2g of oxygen.
To find the molar mass, you'll have to perform dimensional analysis (process of using relationships to translate units you have into units you want) to find an answer that is in the form of grams per mole (g/mol). The unit of molar mass is g/mol.
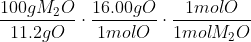
Since you know that there is 11.2g O in 100g of M2O, 16.00g O in every 1 mol of O, and 1 mol of O in every 1 mol M2O, you can multiply out the equation above and you'll find that the answer's units is in the form of
You should receive an output of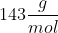 M2O.
M2O.
Because 143 is the molar mass of M2O, to find the molar mass of the metal, subtract 16 (molar mass of O) to get 127g/mol.
If 127g/mol corresponds to M2, then...
127/2 or 63.5g/mol corresponds to M (molar mass of the metal)!
Algebraic shortcut:
if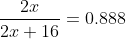
which describes the percent composition of the metal by comparing the amount of the mass of M2 to the total mass of M2O
where x is the molar mass of M, you'll see that x=63.4g/mol...
Hope this helps (:
To find the molar mass, you'll have to perform dimensional analysis (process of using relationships to translate units you have into units you want) to find an answer that is in the form of grams per mole (g/mol). The unit of molar mass is g/mol.
Since you know that there is 11.2g O in 100g of M2O, 16.00g O in every 1 mol of O, and 1 mol of O in every 1 mol M2O, you can multiply out the equation above and you'll find that the answer's units is in the form of
You should receive an output of
Because 143 is the molar mass of M2O, to find the molar mass of the metal, subtract 16 (molar mass of O) to get 127g/mol.
If 127g/mol corresponds to M2, then...
127/2 or 63.5g/mol corresponds to M (molar mass of the metal)!
Algebraic shortcut:
if
which describes the percent composition of the metal by comparing the amount of the mass of M2 to the total mass of M2O
where x is the molar mass of M, you'll see that x=63.4g/mol...
Hope this helps (:
Return to “Empirical & Molecular Formulas”
Who is online
Users browsing this forum: No registered users and 3 guests

