For Ms (spin up, spin down)
Moderators: Chem_Mod, Chem_Admin
-
Cooper_Geralds_3B
- Posts: 100
- Joined: Wed Sep 30, 2020 10:07 pm
For Ms (spin up, spin down)
For the value of Ms, is there an arrow always given to indicate whether it is spin up or spin down or is there another way to determine whether the value is + or -1/2?
-
Jordan Tatang 3L
- Posts: 125
- Joined: Wed Sep 30, 2020 9:31 pm
- Been upvoted: 3 times
Re: For Ms (spin up, spin down)
I think the main notation for this would just be the up/down arrows in the orbitals.
-
Courtney Situ 2B
- Posts: 112
- Joined: Wed Sep 30, 2020 10:03 pm
Re: For Ms (spin up, spin down)
Hi there!
I thought you were asking if the +1/2 and -1/2 corresponded to a specific arrow (up/down). Generally, I believe the up arrow, which corresponds to spin up, represents a spin number of +1/2. Therefore, the down arrow, which corresponds to spin down, represents a spin number of -1/2.
Hope that helps!
I thought you were asking if the +1/2 and -1/2 corresponded to a specific arrow (up/down). Generally, I believe the up arrow, which corresponds to spin up, represents a spin number of +1/2. Therefore, the down arrow, which corresponds to spin down, represents a spin number of -1/2.
Hope that helps!
-
Andrew Yoon 3L
- Posts: 102
- Joined: Wed Sep 30, 2020 9:36 pm
Re: For Ms (spin up, spin down)
For +1/2, I think it is supposed to represent spin up, and -1/2 is supposed to represent spin down.
-
Mari Williams 1K
- Posts: 104
- Joined: Wed Sep 30, 2020 9:53 pm
Re: For Ms (spin up, spin down)
Do we ever use 0? Is there ever a situation in which an electron has no spin?
-
Jonathan Banh 1G
- Posts: 60
- Joined: Wed Sep 30, 2020 9:33 pm
Re: For Ms (spin up, spin down)
I believe that the only values for the spin quantum number are 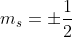 . When determining the value for
. When determining the value for 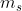 , this is denoted by the arrows in the orbitals, where if they are pointing upwards, the spin is 1/2 while if they are pointing downwards, the spin is -1/2. On a side note, it is important to remember that electrons tend to fill up all the orbitals before pairing up together, which can affect your value of
, this is denoted by the arrows in the orbitals, where if they are pointing upwards, the spin is 1/2 while if they are pointing downwards, the spin is -1/2. On a side note, it is important to remember that electrons tend to fill up all the orbitals before pairing up together, which can affect your value of 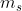 .
.
-
Jack_Pearce_2H
- Posts: 110
- Joined: Wed Sep 30, 2020 9:48 pm
Re: For Ms (spin up, spin down)
It's usually just an arrow unless otherwise stated (i.e. if the questions says that another electron with the same first three quantum numbers and an Ms of +1/2 is in an orbital, you know the other electron will have -1/2).
Return to “*Shrodinger Equation”
Who is online
Users browsing this forum: No registered users and 4 guests

