I believe the correct answer is C)
Post-Module Assessment typo?? - Balancing Chemical Reactions
Moderators: Chem_Mod, Chem_Admin
-
Jessica Dharmawan 1G
- Posts: 32
- Joined: Fri Sep 28, 2018 12:15 am
Post-Module Assessment typo?? - Balancing Chemical Reactions
On question #17, it asks us to balance the equation  .
.
I believe the correct answer is C)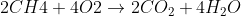 , but the survey says it is wrong, maybe it's a typo?
, but the survey says it is wrong, maybe it's a typo?
I believe the correct answer is C)
-
Sydney To 1D
- Posts: 60
- Joined: Fri Sep 28, 2018 12:15 am
Re: Post-Module Assessment typo?? - Balancing Chemical Reactions
There is no typo. The correct answer is D) +2O{_{2}}(g)\rightarrow CO{_{2}}(g)+2H{_{2}}O(g)) . Answer choice D is the best answer choice for the balanced equation as the ratio of moles (the stoichiometric coefficients) are presented in the lowest terms.
. Answer choice D is the best answer choice for the balanced equation as the ratio of moles (the stoichiometric coefficients) are presented in the lowest terms.
-
Ester Garcia 1F
- Posts: 29
- Joined: Fri Sep 28, 2018 12:17 am
Re: Post-Module Assessment typo?? - Balancing Chemical Reactions
The correct balanced equation would be CH4+2O2>CO2+2H2O not 2CH4+4O2>2CO2+4H2O. The coefficients of the equation can be further simplified by dividing by 2 therefore the balanced equation has a ratio of 1:2:1:2.
-
Michael Nirula
- Posts: 32
- Joined: Fri Sep 28, 2018 12:27 am
Re: Post-Module Assessment typo?? - Balancing Chemical Reactions
Prof. Lavelle mentioned that balancing rxns is just like solving math equations so if you left your answer as is it would be like saying 2x=2 when you really should simplify to x=1 if that makes sense.
Return to “Balancing Chemical Reactions”
Who is online
Users browsing this forum: No registered users and 7 guests

