L.5 Part B Sixth Edition [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Albert_Luu3K
- Posts: 62
- Joined: Fri Sep 28, 2018 12:23 am
L.5 Part B Sixth Edition
I can't seem to get the correct answer for part b. I converted 3.500x10^3 kg Al to g and then to moles by dividing by its molar mass. Then I used molar ratios to find out how many moles of Al2O3 is made, turned that to g and then to kg. My answer doesn't seem to match. Am I making any wrong steps?
-
Rachana Jayaraman 1H
- Posts: 61
- Joined: Fri Sep 28, 2018 12:26 am
Re: L.5 Part B Sixth Edition
Your steps are correct. I would recommend double checking your calculations at each step to make sure you did not enter any numbers incorrectly. You could also try doing all the calculations at once like in the solutions manual:
(3500 kg Al)(1000 g Al/1 kg Al)(1 mol Al/26.98 g Al)(5 mol Al2O3/10 mol Al)(101.96 g Al2O3/1 mol Al2O3)
(3500 kg Al)(1000 g Al/1 kg Al)(1 mol Al/26.98 g Al)(5 mol Al2O3/10 mol Al)(101.96 g Al2O3/1 mol Al2O3)
-
yaosamantha4F
- Posts: 31
- Joined: Fri Sep 28, 2018 12:29 am
Re: L.5 Part B Sixth Edition
Your steps look right; maybe double check that you're using the right molar masses and inputting the correct numbers into your calculator?
-
Sarah Bui 2L
- Posts: 61
- Joined: Fri Sep 28, 2018 12:29 am
Re: L.5 Part B Sixth Edition
Here is my work for b, maybe your ratios were incorrect? Hope this helps!
-
Joon Chang 2F
- Posts: 59
- Joined: Fri Sep 28, 2018 12:25 am
Re: L.5 Part B Sixth Edition
The answer is in the book is in g. Other than your final step of converting g to kg, your steps are correct.
3.500x10^3 kg * (1000g/1kg) = 3.500x10^6 g
3.500x10^6 g * (1mol Al/26.982g) = 129716.107 mol Al
129716.107 mol Al *(5mol Al2O3 / 10mol Al) = 64858.054 mol Al2O3
64858.054 mol Al2O3 * (101.961g Al2O3 / 1mol Al2O3) = 6612991.995 g Al2O3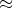 6.613x10^6 g Al2O3
6.613x10^6 g Al2O3
3.500x10^3 kg * (1000g/1kg) = 3.500x10^6 g
3.500x10^6 g * (1mol Al/26.982g) = 129716.107 mol Al
129716.107 mol Al *(5mol Al2O3 / 10mol Al) = 64858.054 mol Al2O3
64858.054 mol Al2O3 * (101.961g Al2O3 / 1mol Al2O3) = 6612991.995 g Al2O3
Re: L.5 Part B Sixth Edition [ENDORSED]
For L.5 Part B, I converted the 3.5x10^3 to grams by multiplying by 1000g. With the value of Aluminum in grams, I divided by 26.98g to get the amount of moles of Al. And given the chemical equation 6NH4ClO4 + 10Al(s) --> 5Al2O3(s) + 3N2(g) + 6HCl(g) + 9H20, I used stoichiometry/mole conversions to convert to Al2O3. So after I found the amount of moles of Al, the ratio of Al to Al2O3 is 10moles (Al): 5moles (Al2O3). Now that we have the value of Al2O3 in moles, we multiply this value by its molar mass and get 101.96g Al2O3 which gives us 6.61x10^6gAl2O3.
I went from kgAl -> gAl -> mol Al -> mol Al2O3 -> g Al2O3
I went from kgAl -> gAl -> mol Al -> mol Al2O3 -> g Al2O3
Return to “Balancing Chemical Reactions”
Who is online
Users browsing this forum: No registered users and 4 guests

