Products of combustion reactions
Moderators: Chem_Mod, Chem_Admin
-
Erik Buetow 1F
- Posts: 96
- Joined: Fri Aug 30, 2019 12:15 am
Products of combustion reactions
Are CO2 and H2O always products in a combustion reaction? If so, can there be any other products also or just those two?
-
Mashkinadze_1D
- Posts: 87
- Joined: Sat Aug 24, 2019 12:15 am
Re: Products of combustion reactions
Hello! In the majority of combustion, oxidation, and metabolizing reactions , carbon dioxide and water will be the products. Although these are usually the products, there may be additional molecular compounds that were also created during the combustion reaction. Hope this helps!
-
nehashetty_2G
- Posts: 102
- Joined: Thu Jul 25, 2019 12:15 am
Re: Products of combustion reactions
I believe combustion reactions always only produce H20 and CO2. I have never seen anything other than that. The only other product other than H20 and CO2 that I can think of would be heat.
-
Andrew Liang 1I
- Posts: 105
- Joined: Fri Aug 30, 2019 12:18 am
Re: Products of combustion reactions
Yes! In complete combustion CO2 and H2O are usually the byproduct of combustion unless the problem gives you other byproduct. However i believe in incomplete combustion, CO is produced instead of CO2.
-
Eesha Sohail 1D
- Posts: 100
- Joined: Sat Aug 17, 2019 12:16 am
Re: Products of combustion reactions
If I recall correctly, incomplete combustion occurs in the presence of inadequate oxygen, but I'd appreciate if someone could check me on that. An example equation I found for the process, ft. methane:
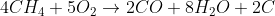
Would this also possibly count as a side reaction? As in, do most combustion reactions go to completion or is incomplete combustion a possible source of experimental error?
Would this also possibly count as a side reaction? As in, do most combustion reactions go to completion or is incomplete combustion a possible source of experimental error?
-
Malia Shitabata 1F
- Posts: 127
- Joined: Sat Aug 17, 2019 12:17 am
-
IScarvie 1E
- Posts: 66
- Joined: Sat Aug 17, 2019 12:16 am
Re: Products of combustion reactions
I believe when you are told to write an equation for a combustion reaction, you assume the products will be CO2 and H2O. However, sometimes you will be given other products, like in homework problem M19.
-
annikaying
- Posts: 94
- Joined: Sat Sep 14, 2019 12:16 am
Re: Products of combustion reactions
The products always include CO2 and H2O but there can also be additional products depending on what is being combusted. For example the combustion of caffeine (C8H10N4O2) also produces the product NO2 alongside CO2 and H2O.
Return to “Balancing Chemical Reactions”
Who is online
Users browsing this forum: No registered users and 2 guests

