combustion reaction
Moderators: Chem_Mod, Chem_Admin
-
jonathan chi 1J
- Posts: 105
- Joined: Fri Sep 24, 2021 6:02 am
-
Bryan Cheng 1H
- Posts: 104
- Joined: Fri Sep 24, 2021 7:03 am
- Been upvoted: 3 times
Re: combustion reaction
I typically start with carbon, since it's trivial to go from whatever hydrocarbon you're given to the amount of CO2. Then I balance the hydrogen, since it only appears in one species on either side (of course, the same can be said of carbon). The final step is to balance oxygen, which is a bit more involved since oxygen appears in both carbon dioxide and water.
For example, in the complete combustion of heptane,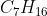 , we know that there is a 1:7 ratio between heptane and carbon dioxide. Then we know that there is a 1:8 ratio between heptane and water (16/2=8). Finally, since there is no oxygen in heptane, add up the oxygen atoms to get 7*2+8=22 atoms or 11 molecules of O2. So we get the balanced equation as:
, we know that there is a 1:7 ratio between heptane and carbon dioxide. Then we know that there is a 1:8 ratio between heptane and water (16/2=8). Finally, since there is no oxygen in heptane, add up the oxygen atoms to get 7*2+8=22 atoms or 11 molecules of O2. So we get the balanced equation as:
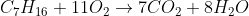
For example, in the complete combustion of heptane,
-
Christina Gigoux 1D
- Posts: 102
- Joined: Fri Sep 24, 2021 5:11 am
Re: combustion reaction
I tend to balance these the same way. In general, it is easiest to balance things that appear the least, then the second least, etc. until everything is balanced.
-
Ellie Fox 2K
- Posts: 100
- Joined: Fri Sep 24, 2021 5:28 am
Re: combustion reaction
I agree with the post above, I think the easiest way to balance equations is to start with balancing the atom that appears the least, then work from there.
Re: combustion reaction
When balancing any chemical equation, the best method is to start by balancing the atom that is the least abundant. That is why we usually balance the number of oxygen atoms at the end because it is usually not the least abundant in the chemical equation.
Re: combustion reaction
I first find the molecules in the equation that are by themselves and balance these elements last because it is easy to change their coefficient without affecting the amount of any other element.
Return to “Balancing Chemical Reactions”
Who is online
Users browsing this forum: No registered users and 3 guests

