Hi,
I was wondering if someone could please explain that if a question asks how an increase in the pressure or a decrease in the volume of a reaction will affect the direction the reaction favors how do you figure it out?
For an example, can someone please explain Fall 2014, Question 6. What conditions favor SO2 production in 2SO<-> 2SO+O2. delta H=+198KJ. Also in this question what is delta H?
And Fall 2015 Question 10: For the reaction at equilibrium HCl+I2<->HI +Cl2, how will an increase in the pressure by decreasing the volume effect the concentration of Cl2?
Thank you.
Ideal Gas Law and Equilibrium Constant
Moderators: Chem_Mod, Chem_Admin
-
Britney Pheng 1L
- Posts: 22
- Joined: Wed Sep 21, 2016 2:56 pm
- Been upvoted: 1 time
Re: Ideal Gas Law and Equilibrium Constant
Increasing pressure (by decreasing the volume) will make your chemical equation favor the side with less gas particles.
Fall 2015 Question 10
So in this problem, you only need to look at the gas molecules (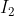 is a solid). After you balance the chemical equation, you get:
is a solid). After you balance the chemical equation, you get:  + I_{2} (s) \leftrightarrow 2HI (g) + Cl_{2} (g).)
There are more gas particles on the side of the products (2 reactants, 3 products). We have an increase in pressure, so to minimize this change in pressure, you would want to move to the side with fewer particles (the reactants side). If we move to the reactants side (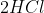 ), there will be a decrease in the concentration of
), there will be a decrease in the concentration of  .
.
Fall 2014 Question 6
For this problem there are 2 reactant particles and 3 product particles. Since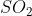 (g) is on the side with more particles, less pressure would be ideal. To favor the side with more gas particles (in this case,
(g) is on the side with more particles, less pressure would be ideal. To favor the side with more gas particles (in this case, 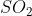 ’s side), there would need to be a decrease in pressure.
’s side), there would need to be a decrease in pressure.
 is the change in enthalpy of the reaction.
is the change in enthalpy of the reaction.
Fall 2015 Question 10
So in this problem, you only need to look at the gas molecules (
There are more gas particles on the side of the products (2 reactants, 3 products). We have an increase in pressure, so to minimize this change in pressure, you would want to move to the side with fewer particles (the reactants side). If we move to the reactants side (
Fall 2014 Question 6
For this problem there are 2 reactant particles and 3 product particles. Since
-
Minu Reddy
- Posts: 31
- Joined: Sat Jul 09, 2016 3:00 am
Re: Ideal Gas Law and Equilibrium Constant
Ok thank you very much.
But isn't this a concept that we have not covered yet?
But isn't this a concept that we have not covered yet?
-
Myra_Zhan_2N
- Posts: 25
- Joined: Fri Jul 22, 2016 3:00 am
Re: Ideal Gas Law and Equilibrium Constant
We didn't really cover it in lecture, but it is part of the section explaining the properties of gases which is something we should know for the quiz.
-
Alexandria_Leaf_2F
- Posts: 30
- Joined: Fri Jul 15, 2016 3:00 am
Chemical Equilibrium Part 3 Module
The equilibrium constant, KP, for the reaction SO2 (g) + O2 (g) ⇌ SO3 (g) at 700 K is 3 x 104. A mixture of SO2, O2, and SO3, each at 65 bars was introduced into a container at 700 K. Is the reaction at equilibrium? If not, does SO3 tend to form or decompose?
-
Alexandria_Leaf_2F
- Posts: 30
- Joined: Fri Jul 15, 2016 3:00 am
Chemical Equilibrium Part 3 Module
A mixture of 2.5 moles H2O and 100 g of C are placed in a 50 L container and allowed to come to equilibrium subject to the following reaction:
C(s) + H2O (g) ⇌ CO (g) + H2 (g)
The equilibrium concentration of hydrogen is found to be [H2] = 0.040 M. What is the equilibrium concentration of water, [H2O]?
C(s) + H2O (g) ⇌ CO (g) + H2 (g)
The equilibrium concentration of hydrogen is found to be [H2] = 0.040 M. What is the equilibrium concentration of water, [H2O]?
-
Alexa_Kwang_1D
- Posts: 24
- Joined: Wed Sep 21, 2016 3:00 pm
Re: Chemical Equilibrium Part 3 Module
Wait how do you solve that second problem without knowing the equilibrium constant?
-
Xiaoman_Kang_2J
- Posts: 19
- Joined: Wed Sep 21, 2016 3:00 pm
Re: Chemical Equilibrium Part 3 Module
Alexa_Kwang_1H wrote:Wait how do you solve that second problem without knowing the equilibrium constant?
For that question, we don't really need to know the equilibrium constant to solve it. If the pressure of the whole system is increased by decreasing the volume, the reaction will proceed to the side with less moles of molecules to decrease the pressure of the system.
Who is online
Users browsing this forum: No registered users and 9 guests

