A 0.253 mol
sample of NO2(g),
initially at 298 K
and 1.00 atm,
is held at constant pressure while enough heat is applied to raise the temperature of the gas by 18.9 K.
Calculate the amount of heat q
required to bring about this temperature change, and find the corresponding total change in the internal energy ΔU
of the gas.
Assume that the constant‑pressure molar specific heat for NO2(g),
which consists of nonlinear molecules, is equal to 4R,
where R=8.3145 J/(mol·K)
is the ideal gas constant.
Can someone help explain the second part of this question, please?
Week 3 and 4 HW
Moderators: Chem_Mod, Chem_Admin
-
Suzy Matinyan
- Posts: 35
- Joined: Mon Jan 09, 2023 10:12 am
Re: Week 3 and 4 HW
Hi!
So I have not done this question yet, but this is how I would approach it and hopefully this should get us the correct answer.
First, to get q I'm assuming you used the equation q = n C delta T and it should just be simply plugging in the information provided for the equation
So the second part, delta U = q + w should be used and we know q from the first part. If q isn't the only thing contributing to this change in internal energy then work might be being done too. So, if mols, pressure and R are constant, but T is changing, then volume must be changing according to PV=nRT. You can calculate the change in volume from this equation and then plug it into w =-PdeltV to find this work and then combine this with q to find delta U. Hopefully this will get the right answer!
So I have not done this question yet, but this is how I would approach it and hopefully this should get us the correct answer.
First, to get q I'm assuming you used the equation q = n C delta T and it should just be simply plugging in the information provided for the equation
So the second part, delta U = q + w should be used and we know q from the first part. If q isn't the only thing contributing to this change in internal energy then work might be being done too. So, if mols, pressure and R are constant, but T is changing, then volume must be changing according to PV=nRT. You can calculate the change in volume from this equation and then plug it into w =-PdeltV to find this work and then combine this with q to find delta U. Hopefully this will get the right answer!
-
samantha eberle 2L
- Posts: 38
- Joined: Mon Jan 09, 2023 9:35 am
Re: Week 3 and 4 HW
I have attached a written ou solution/calculation.
For the second part of this problem, since it states that there is constant pressure during this process, we can assume that the volume must be changing. But since we are not given the change in volume for this problem, calculating the work can be a but confusing. We know that delta U = q + w for a system, and that our work can be found through the equation w = -P * deltaV. if we relate this to the ideal gas law PV=nRT, we see that work is also equal to -nRT. so we can plug this in instead.
Thus,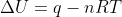 for this problem.
for this problem.
For the second part of this problem, since it states that there is constant pressure during this process, we can assume that the volume must be changing. But since we are not given the change in volume for this problem, calculating the work can be a but confusing. We know that delta U = q + w for a system, and that our work can be found through the equation w = -P * deltaV. if we relate this to the ideal gas law PV=nRT, we see that work is also equal to -nRT. so we can plug this in instead.
Thus,
-
Anahi C 2F
- Posts: 34
- Joined: Mon Jan 09, 2023 9:09 am
Re: Week 3 and 4 HW
Second part (solving for deltaU):
As heat is added to the gas, some of the energy goes into the work done by the gas as it expands. The rest of the energy goes into the internal energy of the gas. Therefore, the change in internal energy (ΔU) that corresponds to a temperature change (deltaT) is given by deltaU=nCV deltaT. In the equation, CV is the constant‑volume molar specific heat of the gas. CV is related to CP by CV= CP −R= 4R−R=3R.
Therefore, ΔU=(0.253 mol)(3)(8.3145Jmol⋅K)(18.9 K)
As heat is added to the gas, some of the energy goes into the work done by the gas as it expands. The rest of the energy goes into the internal energy of the gas. Therefore, the change in internal energy (ΔU) that corresponds to a temperature change (deltaT) is given by deltaU=nCV deltaT. In the equation, CV is the constant‑volume molar specific heat of the gas. CV is related to CP by CV= CP −R= 4R−R=3R.
Therefore, ΔU=(0.253 mol)(3)(8.3145Jmol⋅K)(18.9 K)
-
rihannasbestfriend
- Posts: 61
- Joined: Mon Jan 09, 2023 8:26 am
Re: Week 3 and 4 HW
Anahi C 2F wrote:Second part (solving for deltaU):
As heat is added to the gas, some of the energy goes into the work done by the gas as it expands. The rest of the energy goes into the internal energy of the gas. Therefore, the change in internal energy (ΔU) that corresponds to a temperature change (deltaT) is given by deltaU=nCV deltaT. In the equation, CV is the constant‑volume molar specific heat of the gas. CV is related to CP by CV= CP −R= 4R−R=3R.
Therefore, ΔU=(0.253 mol)(3)(8.3145Jmol⋅K)(18.9 K)
Wait how are you getting deltaU=nCVdeltaT for internal energy equation
Who is online
Users browsing this forum: No registered users and 5 guests

