Interaction Potential Energy
Moderators: Chem_Mod, Chem_Admin
-
Zoya Mulji 1K
- Posts: 105
- Joined: Thu Jul 11, 2019 12:15 am
Interaction Potential Energy
Can someone explain the components of the formula for interaction potential energy and what the alpha and r means?
-
Charisse Vu 1H
- Posts: 101
- Joined: Thu Jul 25, 2019 12:17 am
Re: Interaction Potential Energy
Alpha represents the polarizability of an atom and depends on the number of electrons and size of the atom. R in the equation represents the radius. So, according to the equation, the interaction potential energy is proportional to 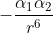 .
.
-
Kaitlyn Ang 1J
- Posts: 116
- Joined: Fri Aug 09, 2019 12:17 am
- Been upvoted: 1 time
Re: Interaction Potential Energy
Also, in context, this means that if the radius between the atoms increases, the denominator gets larger, meaning that the interaction potential energy gets less negative (so it has stronger attractive interactions).
-
chrischyu4a
- Posts: 52
- Joined: Thu Jul 25, 2019 12:16 am
Re: Interaction Potential Energy
alpha represents the polarizability of the atom which correlates with the amount of electrons that are present in the molecule. This is because more electrons allow for more distribution around the molecule which can create stronger momentary dipoles between other molecules. r represents the distance between two molecules and as shown through the equation, as the distance decreases, the greater the potential energy. This is because there is a stronger effective nuclear charge the closer adjacent molecules are to each other.
Who is online
Users browsing this forum: No registered users and 9 guests

