Work of Expansion [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Marc Farah 3D
- Posts: 22
- Joined: Wed Sep 21, 2016 2:59 pm
Work of Expansion
For Work of expansion, why must we measure the changes in energy for in very small steps? And therefore having to use an integral for the infinitesimal changes in volume? When do we use w=-pV as opposed to the integral equation for w?
Re: Work of Expansion [ENDORSED]
I'm not sure if this anywhere near what answer you were looking for, but ..
I think for reactions that happen quickly (at infinitesimal changes), we can use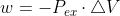 . For reactions that happen more slowly, we use the integral (which is the cumulative sum of all the
. For reactions that happen more slowly, we use the integral (which is the cumulative sum of all the 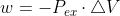 at each infinitesimal change/each stage of expansion). In the end, they both would give approximately the same answer.
at each infinitesimal change/each stage of expansion). In the end, they both would give approximately the same answer.
In our problems, the book usually compares the amount of work between a irreversible path and the reversible and isothermal path.
We use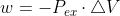 because the an irreversible path indicates that there is the gradual reduction of force in external pressure that changes the opposing force, making the potential of the system to do work less than what is maximally possible.
because the an irreversible path indicates that there is the gradual reduction of force in external pressure that changes the opposing force, making the potential of the system to do work less than what is maximally possible.
For the reversible and isothermal path, the pressure of gas falls as it expands, so, to be reversible, the external pressure must fall as the volume expands so that at every stage of expansion, the external pressure matches with the pressure of the gas (which makes the potential of work at every stage of expansion done maximally).
I think for reactions that happen quickly (at infinitesimal changes), we can use
In our problems, the book usually compares the amount of work between a irreversible path and the reversible and isothermal path.
We use
For the reversible and isothermal path, the pressure of gas falls as it expands, so, to be reversible, the external pressure must fall as the volume expands so that at every stage of expansion, the external pressure matches with the pressure of the gas (which makes the potential of work at every stage of expansion done maximally).
-
Christine_Mavilian_3E
- Posts: 30
- Joined: Fri Jul 22, 2016 3:00 am
Re: Work of Expansion
If I correctly understand your question, based on the lecture today, there was an example with a diagram where the system (ideal gas) was expanding to twice its volume. The mass would slowly decrease and the system would expand slowly, doing more work. During such process it will lose energy but it's in thermal equilibrium so whatever energy lost will be replaced by heat going into the system from the surroundings at constant temperature. If the process is done slowly the energy change will be minimal and the temperature will remain constant (isothermal).
You would use w=-pv when the pressure is constant. You would use the integral which we learned today that after simplifying equals w=-nRTln (V2/V1) when pressure changes at the same time as the volume; in other words, when work done by the system is not equal.
I hope this helps!
You would use w=-pv when the pressure is constant. You would use the integral which we learned today that after simplifying equals w=-nRTln (V2/V1) when pressure changes at the same time as the volume; in other words, when work done by the system is not equal.
I hope this helps!
Return to “Thermodynamic Systems (Open, Closed, Isolated)”
Who is online
Users browsing this forum: Google [Bot] and 12 guests

