Van't Hoff Equation
Moderators: Chem_Mod, Chem_Admin
-
Alyssa Wilson 2A
- Posts: 65
- Joined: Fri Sep 28, 2018 12:18 am
Van't Hoff Equation
I'm a little confused on why the Van't Hoff equation shows the temperature dependence of K. I understand that it relates the difference in standard Gibbs free energy to K, because it's easy to measure, but why does it show that the temperature depends on K?
Re: Van't Hoff Equation
The Van't Hoff equation is meant to show the Temperature dependance of K, and while it is derived from an expression that relates G to K, in its ultimate for the equation excludes G.
Derivation:
1) Given) and
and 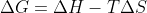 :
:
)
2) Divide both sides by -RT to isolate ln(K):
 = \frac{-\Delta H}{RT} +\frac{\Delta S}{R})
3) Use the above equation, plug in K1 at T1 to make the equation for initial K and plug in K2 at T2 to make the equation for Final K. Subtract the final K equation by initial K equation:
 - ln(K_{1}) = \frac{-\Delta H}{RT_{2}} + \frac{\Delta H}{RT_{1}} +\frac{\Delta S}{R} -\frac{\Delta S}{R})
4) Simplify the equation by using ln(a)-ln(b) = ln(a/b) and cancelling out the entropy terms:
 = \frac{-\Delta H}{RT_{2}} +\frac{\Delta H}{RT_{1}})
5) Now you factor out -delta H/R to get the final form that we use:
 = \frac{-\Delta H}{R}(\frac{1}{T_{2}}-\frac{1}{T_{1}}))
Note how the process of deriving this equation eventually allows us to compute a final K @ Tfinal from an initial K @ Tini knowing only the enthalpy of reaction.
Derivation:
1) Given
2) Divide both sides by -RT to isolate ln(K):
3) Use the above equation, plug in K1 at T1 to make the equation for initial K and plug in K2 at T2 to make the equation for Final K. Subtract the final K equation by initial K equation:
4) Simplify the equation by using ln(a)-ln(b) = ln(a/b) and cancelling out the entropy terms:
5) Now you factor out -delta H/R to get the final form that we use:
Note how the process of deriving this equation eventually allows us to compute a final K @ Tfinal from an initial K @ Tini knowing only the enthalpy of reaction.
Return to “Thermodynamic Systems (Open, Closed, Isolated)”
Who is online
Users browsing this forum: No registered users and 8 guests

