Isolated// Energy
Moderators: Chem_Mod, Chem_Admin
-
Daniel Honeychurch1C
- Posts: 109
- Joined: Thu Jul 11, 2019 12:15 am
Re: Isolated// Energy
In an isolated system, matter and energy cannot be exchanged between the system and the environment. Therefore, the total energy in an isolated system cannot change.
-
Anthony Hatashita 4H
- Posts: 103
- Joined: Wed Sep 18, 2019 12:21 am
Re: Isolated// Energy
There can be reactions that happen within the isolated system, but no energy can leave or enter the system.
-
Sebastian Lee 1L
- Posts: 157
- Joined: Fri Aug 09, 2019 12:15 am
- Been upvoted: 1 time
Re: Isolated// Energy
An isolated system has no transfer of matter or energy.
From the equation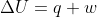 , it should make sense that the change in internal energy of an isolated system will be 0 because there is no heat transfer (energy) and no work being done (also energy).
, it should make sense that the change in internal energy of an isolated system will be 0 because there is no heat transfer (energy) and no work being done (also energy).
From the equation
-
Eva Zhao 4I
- Posts: 101
- Joined: Sun Sep 29, 2019 12:16 am
Re: Isolated// Energy
No, in an isolated system there can be no exchange of energy with the surrounding. The combustion of glucose in a bomb calorimeter is an example. To clarify, a closed system can have energy exchange with surroundings by heating/cooling or compression/expansion.
Re: Isolated// Energy
No because it cannot exchange energy with the surrounding environment because it is closed off.
Re: Isolated// Energy
For the system as a whole, there cannot be an exchange in energy because its isolated so the energy is constant.
-
Maika Ngoie 1B
- Posts: 97
- Joined: Fri Aug 02, 2019 12:16 am
Re: Isolated// Energy
No, neither energy nor matter can be transferred between a system and surroundings in an isolated system.
Re: Isolated// Energy
Energy of a isolated system is always conserved (1st law), so even in an isolated system as big as the entire universe, the overall change in energy is 0
-
Andrea_Torres
- Posts: 98
- Joined: Sun Sep 15, 2019 12:15 am
Re: Isolated// Energy
Since an isolated system has no exchanges with its surroundings, this means that the isolated system can not have a change in energy.
-
Veronica Lu 2H
- Posts: 89
- Joined: Wed Sep 18, 2019 12:18 am
- Been upvoted: 1 time
Re: Isolated// Energy
There is no change in energy in an isolated system since there is no interaction with the outside environment
Re: Isolated// Energy
No, because the definition of an isolated system is that no energy is exchanged with the environment. This includes heat and work. Consider it like a thermally-insulated and rigid bomb calorimeter.
Return to “Thermodynamic Systems (Open, Closed, Isolated)”
Who is online
Users browsing this forum: No registered users and 5 guests

