Delta in PV=nRT
Moderators: Chem_Mod, Chem_Admin
-
Karrin Evans 1C
- Posts: 34
- Joined: Mon Jan 09, 2023 2:28 am
- Been upvoted: 1 time
Delta in PV=nRT
In class, we discussed that 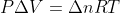 , based on the ideal gas law. Does the delta apply just to n, or to the whole nRT term? And why?
, based on the ideal gas law. Does the delta apply just to n, or to the whole nRT term? And why?
Re: Delta in PV=nRT
The delta only applies to n, or the value of moles because in the case discussed in the lecture, the pressure and temperature are constant. Therefore, only the volume and moles are the only factors changing.
-
Nicholas Flores 2F
- Posts: 34
- Joined: Mon Jan 09, 2023 9:10 am
Re: Delta in PV=nRT
In Delta n (RT), the delta only applies to the number of moles (n) because R is a constant, and T and P must be constant in order to use this method. Because pressure is constant, the volume must change, meaning that delta-V must have a value. Also, because there is a volume change, there must be a change in the moles of the chemical reaction as the number of moles changes the volume and pressure of the system (and since pressure is constant volume must change). Hope this helps!
-
mieke van daelen 2I
- Posts: 40
- Joined: Mon Jan 09, 2023 9:24 am
Re: Delta in PV=nRT
The use of this equation relies on a constant pressure and temperature, and R itself is a constant (can't change) so, therefore the delta in the right-hand side of the equation only applies to n, the number of moles.
-
kamrin schultz 2J
- Posts: 35
- Joined: Mon Jan 09, 2023 9:25 am
Re: Delta in PV=nRT
The delta on the right-hand side of the equation is only referring to the change in the number moles. P, R, and T are all constant.
-
Varchas Bharadwaj 2G
- Posts: 40
- Joined: Mon Jan 09, 2023 9:13 am
Re: Delta in PV=nRT
The delta will only apply to V and n, since pressure & temperature are assumed to be constant, while R is itself a constant.
-
Daniel Adolfo 2L
- Posts: 34
- Joined: Mon Jan 09, 2023 9:37 am
Re: Delta in PV=nRT
Given that pressure, temperature, and R are assumed to be constant in this case, the equation is only asking for the change in the amount of moles. Hope this helps.
-
suhas yarra 2D
- Posts: 34
- Joined: Mon Jan 09, 2023 8:57 am
Re: Delta in PV=nRT
The delta only applies to n and V. This is because R is the ideal gas constant and Pressure and temperature are presumed to be constant.
-
Sydney Tanimitsu 2B
- Posts: 34
- Joined: Mon Jan 03, 2022 9:11 am
Re: Delta in PV=nRT
In this case, pressure has to be constant and R and T would also not change. The only variable that can change is volume and in order for the volume, there must be a change in (n) or the moles as well.
-
Emily_Hanna_2E
- Posts: 42
- Joined: Mon Jan 09, 2023 9:03 am
Re: Delta in PV=nRT
Hi Karrin! The change on that side of the equation is related to the moles since R is always a constant, and the Temperature must be constant for us to use this equation. In the example we did in class, R and T were constant and we assumed that the pressure was 1. Given that the open beaker produces a NET of 8 moles (emphasis on the NET) we solved for the change in pressure by plugging in 8 for the change in moles. So, on the left side, the change only applies to the volume and on the right is applies to only the moles.
-
Hamna_Khan_2E
- Posts: 35
- Joined: Mon Jan 09, 2023 9:03 am
Re: Delta in PV=nRT
Hello!
There is only change for the number of moles in the equation. The other components consisting of temperature, pressure, and R are all considered constants.
Hope this helps!
There is only change for the number of moles in the equation. The other components consisting of temperature, pressure, and R are all considered constants.
Hope this helps!
-
Jason T 1I
- Posts: 35
- Joined: Mon Jan 09, 2023 8:27 am
Re: Delta in PV=nRT
Delta will only apply to volume and moles dude to the fact that temperature and pressure are being held constant. This highlights that the only factors that are changing in this equation are the moles and the volume.
-
Luke Iwai 3L
- Posts: 57
- Joined: Mon Jan 09, 2023 10:16 am
-
Oscar Tan 1L
- Posts: 40
- Joined: Mon Jan 09, 2023 8:42 am
Re: Delta in PV=nRT
The delta refers only to moles which can help with solving PdeltaV which is equivalent to work since R and T would be constants. PdeltaV can also be solved when moles are constant and Temperature changes. Just make sure the units are correct for R and the other terms.
-
Skylar Smith 1E
- Posts: 35
- Joined: Wed Feb 17, 2021 12:16 am
Re: Delta in PV=nRT
The delta only applies to the n and the P because it is the overall change of pressure and the moles in the system. In this case the R and T are both constants and given in the equation, so the delta on the n would only apply to the moles.
-
Audric Banuelos 1A
- Posts: 43
- Joined: Mon Jan 09, 2023 2:20 am
Re: Delta in PV=nRT
The delta only applies to moles (n) and not R and T because R and T are constants, therefore, the only thing that changes are the moles. In an example that he showed in class, the problem stated the amount of moles, so in that case, you would input the value for n. R will also be given since it is a constant and T as well, or you can just memorize them.
Re: Delta in PV=nRT
Hello!
The delta applies to the parameter ‘n’ (which is the number of moles) because it’s assumed that ‘P’, ‘R’, and ‘T’ is constant (in an open beaker). So technically, it is P∆V=∆nRT (in an open beaker), since the pressure, temperature, and ‘R-value’ is constant. Then, the change in the number of moles causes a change in volume, so an increase in the number of moles leads to an increase in volume.
The delta applies to the parameter ‘n’ (which is the number of moles) because it’s assumed that ‘P’, ‘R’, and ‘T’ is constant (in an open beaker). So technically, it is P∆V=∆nRT (in an open beaker), since the pressure, temperature, and ‘R-value’ is constant. Then, the change in the number of moles causes a change in volume, so an increase in the number of moles leads to an increase in volume.
Return to “Thermodynamic Systems (Open, Closed, Isolated)”
Who is online
Users browsing this forum: No registered users and 4 guests

