isothermal system
Moderators: Chem_Mod, Chem_Admin
-
Tiffany Cao 1D
- Posts: 94
- Joined: Fri Sep 29, 2017 7:03 am
- Been upvoted: 1 time
isothermal system
When a system is isothermal, does that mean that temperature is constant? Why does this also mean that change in internal energy is also 0?
-
Andres Reynoso 1J
- Posts: 30
- Joined: Fri Sep 29, 2017 7:06 am
Re: isothermal system
Yes, when a system is isothermal it means that there is no temperature change in the system and thus the temperature would be constant. Only for ideal gases does this mean that the change in internal energy is 0 because, for ideal gases, 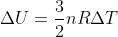 and
and 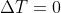 (Anything multiplied by 0 equals 0).
(Anything multiplied by 0 equals 0).
-
Sarah Maraach 2K
- Posts: 31
- Joined: Thu Jul 27, 2017 3:00 am
Re: isothermal system
A good way to remember the types of systems is to break apart the word, so iso: same and thermal: temperature-related. Since the temperature is the same throughout the reaction, it would follow that Tf - Ti would equal 0. Then, in calculation, your change in internal energy would also be 0.
-
Yea Eun Lee 1H
- Posts: 33
- Joined: Fri Sep 29, 2017 7:06 am
Re: isothermal system
But when an ideal gas expands isothermally, volume increases and pressure decreases. Since volume increased, the system did work on the surroundings...so wouldn't work be non-zero, making the change in internal energy non-zero as well?
Re: isothermal system
Internal energy is a state function, but work is not. Therefore, how the change in internal energy occurred doesn't matter since it will always be 0 in isothermal system. However, the path that work takes is important and needs to be calculated, since it doesn't always equal 0 in an isothermal system. the only thing we know for sure is that q=-w in isothermal system.
Return to “Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)”
Who is online
Users browsing this forum: No registered users and 4 guests

