Isobaric systems
Moderators: Chem_Mod, Chem_Admin
Isobaric systems
Which equations do you tend to use when solving for an isobaric system?
Also which values are equal to zero in an isobaric system?
Also which values are equal to zero in an isobaric system?
-
Nathan Nakaguchi 1G
- Posts: 102
- Joined: Wed Sep 18, 2019 12:22 am
- Been upvoted: 1 time
Re: Isobaric systems
When a system is isobaric that means that the pressure is constant, which implies that 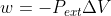 . I think this is all you can assume from this statement. Dr. Lavelle didn't really talk specifically about the terms isobaric and isochoric, so I wouldn't worry too much about it.
. I think this is all you can assume from this statement. Dr. Lavelle didn't really talk specifically about the terms isobaric and isochoric, so I wouldn't worry too much about it.
-
Maya Beal Dis 1D
- Posts: 100
- Joined: Sat Aug 17, 2019 12:16 am
- Been upvoted: 2 times
Re: Isobaric systems
Isobaric is constant pressure, isochoric is constant volume, and isothermal is constant temperature.
-
Max Madrzyk Dis 4G
- Posts: 102
- Joined: Wed Sep 18, 2019 12:21 am
Re: Isobaric systems
Isobaric means constant pressure and when you have a system at constant pressure you can say that delta h=q.
Re: Isobaric systems
In an isobaric system, pressure is constant, so delta P would equal zero. In this case, work can be calculated using the formula w=-PdeltaV and heat can be calculated using q=deltaH.
-
Gabriel Ordonez 2K
- Posts: 113
- Joined: Sat Jul 20, 2019 12:15 am
Re: Isobaric systems
Isobaric is constant pressure, isochoric is constant volume, and isothermal is constant temperature. With these conditions, you'll need to match up the right equations with each one. There are reversible and irreversible equations.
-
Leslie Almaraz 4G
- Posts: 99
- Joined: Fri Aug 02, 2019 12:16 am
-
Amanda Lin 2I
- Posts: 101
- Joined: Sat Aug 17, 2019 12:15 am
-
Kallista McCarty 1C
- Posts: 212
- Joined: Wed Sep 18, 2019 12:18 am
-
Orrin Zhong 4G
- Posts: 51
- Joined: Sat Jul 20, 2019 12:16 am
Re: Isobaric systems
Isobaric means constant pressure. Under these conditions, h = deltaH, since deltaH is defined at constant pressure. Also, w = -(Pext)(deltaV) since pressure is fixed.
-
Jacob Motawakel
- Posts: 103
- Joined: Wed Sep 18, 2019 12:20 am
- Been upvoted: 1 time
-
CMaduno_1L
- Posts: 102
- Joined: Wed Sep 18, 2019 12:18 am
Re: Isobaric systems
During Lyndon and Matt's Pizza Rolls review session, Matt made a comment that might help with remembering the meaning of isobaric. He emphasized the BAR in isoBARic and compared it to BARometer, which is an instrument used to measure atmospheric pressure. Hope this helps!
-
Rebekah Alfred 1J
- Posts: 102
- Joined: Thu Jul 11, 2019 12:15 am
- Been upvoted: 1 time
Re: Isobaric systems
Leslie Almaraz 4G wrote:What about an adiabatic system? is there a different equation for that?
In an adiabatic system, there is no heat transfer (q=0).
-
CalvinTNguyen2D
- Posts: 102
- Joined: Thu Jul 25, 2019 12:16 am
Re: Isobaric systems
Isobaric means constant pressure. Isochoric means constant volume. Isothermal means constant temperature.
-
William Francis 2E
- Posts: 101
- Joined: Wed Sep 18, 2019 12:20 am
Re: Isobaric systems
When pressure is constant, ΔH=q. This is because ΔH=ΔU+PΔV. Since ΔU=q+w, the equation can be rewritten as, ΔH=q+w+PΔV. Since w=-PexΔV, that equation can be rewritten as ΔH=q-PexΔV+PΔV. P=Pex in isobaric conditions so ΔH=q. Therefore, Cp=ΔH/ΔT under these conditions.
-
Kylie Lim 4G
- Posts: 110
- Joined: Sat Aug 17, 2019 12:15 am
Re: Isobaric systems
For isobaric reactions, pressure is constant. This means that both equations for w can be used, as well as the relationship where delta H = q
Re: Isobaric systems
Isobaric systems are systems with constant pressure so you would use an equation that works with the variables you are given as long as pressure is constant. A common equation is deltaH = q
Return to “Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)”
Who is online
Users browsing this forum: No registered users and 9 guests

