ΔH
Moderators: Chem_Mod, Chem_Admin
-
Andrew Yoon 3L
- Posts: 102
- Joined: Wed Sep 30, 2020 9:36 pm
ΔH
In lecture #14, Lavelle went over the relationship between ΔS with T(temp). What did he mean when he said that "the ΔH(rxn) plays an important role at low temperatures"? Can someone clarify how it relates back to the relationship between ΔS and T?
Last edited by Andrew Yoon 3L on Sun Feb 14, 2021 8:33 pm, edited 1 time in total.
-
Rich Luong 1D
- Posts: 101
- Joined: Wed Sep 30, 2020 9:49 pm
Re: ΔH
If you're referring to the Gibbs free energy, then yes, ΔH would play a big role at low temperatures. If you recall, the equation for Gibbs free energy is
ΔG = ΔH - TΔS. If in this case temperature was a very low value, then the significance of ΔS also goes down alongside it. As a result, all you're basically left with is ΔH and because of that, depending on the sign of ΔH, it determines the sign of ΔG.
ΔG = ΔH - TΔS. If in this case temperature was a very low value, then the significance of ΔS also goes down alongside it. As a result, all you're basically left with is ΔH and because of that, depending on the sign of ΔH, it determines the sign of ΔG.
-
Jeffrey Doeve 2I
- Posts: 110
- Joined: Wed Sep 30, 2020 9:51 pm
Re: ΔH
Hi Andrew, I believe that Lavelle was referencing the importance of delta H in comparison to delta S for the Gibbs Free Energy Equation(delta G = delta H - T* delta S). For example, if the T was low, that means that the impact of delta S would be low as well. This means that delta H would dominate and determine whether the reaction is spontaneous(sign of delta G).
-
allyssa bradley 1H
- Posts: 95
- Joined: Wed Sep 30, 2020 9:47 pm
Re: ΔH
Agreeing with the above replies, in a more abstract approach, I think it would be difficult to store energy with respect to the "order" of the system since those particles aren't moving around very much, so even if there are a lot of "occupy-able" states related to entropy and degeneracy, the system is so SLOW that it sort of relies on heat and any work/pressure to store its energy, which would of course be enthalpy.
-
Jaden Joodi 3J
- Posts: 104
- Joined: Wed Sep 30, 2020 9:31 pm
Re: ΔH
From the math point of view, the reason that ΔH plays such a large role at low temperatures is because it is being divided by temperature. The difference between being divided by 1, 2, 3 is quite large compared to being divided by 50,51, 52.
-
Ethan Laureano 3H
- Posts: 102
- Joined: Wed Sep 30, 2020 9:58 pm
Re: ΔH
If you recall the sapling homework, if both delta H and delta S are positive, then the equation would be deltaG = deltaH - TdeltaS. In order to label a reaction spontaneous, deltaG must be negative. At low temperatures, -TdeltaS might not have a high enough magnitude to overcome deltaH and result in a negative deltaG, depending on the magnitude of deltaH.
-
Brennan McGurrr 3C
- Posts: 100
- Joined: Wed Sep 30, 2020 9:47 pm
Re: ΔH
It's due to the equation to calculate Gibbs Free Energy. When T is very low, it will lower the term in the equation with T and delta S. This makes delta G way more dependent on delta H.
-
Ranen_Chang_2G
- Posts: 44
- Joined: Wed Nov 25, 2020 12:21 am
Re: ΔH
You could also look at the relationship S of surroundings and H of reaction have, 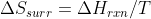 . At low T,
. At low T,  influences the value of
influences the value of 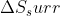 more than T does.
more than T does.
-
Susanna Givan 2B
- Posts: 144
- Joined: Thu Feb 27, 2020 12:16 am
Re: ΔH
Delta S has a very important relationship with temperature--as the temp. increases, the number of particles that can strike each other increases, and the entropy increases. If temp. is very low, then Delta S is effectively zero or very small. Then, the value of Delta H would effectively control Delta G and change it's value.
-
Nandini_Parmar_1I
- Posts: 96
- Joined: Fri Sep 24, 2021 7:14 am
Re: ΔH
The change in enthalpy relates to the change in entropy times the temperature. ie. change in heat minus the temperature difference times the disorder gives the free energy. Gibb's free energy is calculated by constant temperature and pressure.
-
Aanjaneyaa
- Posts: 51
- Joined: Tue Feb 02, 2021 12:17 am
Re: ΔH
Due to the equation deltaG=deltaH-TdeltaS, it shows that both delta S and delta T have an effect on delta G.
-
Caitlin_Doak_2H
- Posts: 52
- Joined: Wed Feb 17, 2021 12:18 am
-
Arshaun Faraji 2H
- Posts: 71
- Joined: Fri Sep 24, 2021 6:42 am
Re: ΔH
it shows that when analyzing the equation deltaG=deltaH-TdeltaS, we should know that deltaH and deltaS are the factors that determine the deltaG
-
Josh Richter 2C
- Posts: 102
- Joined: Fri Sep 24, 2021 5:54 am
Re: ΔH
Those values relate to Gibbs free energy, where they can see whether or not a reaction will be spontaneous
-
raynebunado
- Posts: 101
- Joined: Fri Sep 24, 2021 6:58 am
Return to “Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)”
Who is online
Users browsing this forum: No registered users and 2 guests

