Hi! I'm sure there are already posts about this but I wanted to ask if you all can list how certain conditions affect the internal energy, entropy, enthalpy, work, etc.
So I know that:
-if the initial and finals states are the same, dU and dS will be 0 because they are state functions
-w=0 in isobaric, isochoric conditions
-dU=0 in isothermal processes
I know there are probably many more but I can't think of them from the top of my head.
Can you all list some other ones? Thank you!!
Thermodynamics Rules/Concepts
Moderators: Chem_Mod, Chem_Admin
-
Yasmina Zaarour 1G
- Posts: 100
- Joined: Wed Sep 30, 2020 9:51 pm
- Been upvoted: 1 time
Re: Thermodynamics Rules/Concepts
- If a reaction is reversible, then  and
and 
-If a reaction is irreversible, then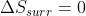 so
so 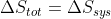
Also would like to add that I think work doesn't equal zero in isobaric (constant pressure) conditions ! w=-P V so it is only equal to zero when it is in isochoric conditions like you added.
V so it is only equal to zero when it is in isochoric conditions like you added.
-If a reaction is irreversible, then
Also would like to add that I think work doesn't equal zero in isobaric (constant pressure) conditions ! w=-P
-
Lakshmi Davuluri 1E
- Posts: 100
- Joined: Wed Sep 30, 2020 9:50 pm
Re: Thermodynamics Rules/Concepts
Yasmina Zaarour 1G wrote:- If a reaction is reversible, thenand
-If a reaction is irreversible, thenso
Also would like to add that I think work doesn't equal zero in isobaric (constant pressure) conditions ! w=-PV so it is only equal to zero when it is in isochoric conditions like you added.
Yeah, I agree with you. I also think that work doesn't equal zero under isobaric conditions because of the equation w=-P*deltaV. Even though pressure is constant, there can still be a change in volume, which would result in a nonzero value for work.
-
MinjooPark_3I
- Posts: 116
- Joined: Wed Sep 30, 2020 9:33 pm
Return to “Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)”
Who is online
Users browsing this forum: No registered users and 9 guests

