finding work with minimal information
Moderators: Chem_Mod, Chem_Admin
-
Jennifer Fimbres
- Posts: 37
- Joined: Wed Feb 15, 2023 8:20 am
finding work with minimal information
When only given internal energy and energy absorbed by external forces, how is work supposed to be calculated, is there another formula besides w=p change of v?
-
Deep Mehta
- Posts: 104
- Joined: Fri Sep 29, 2023 11:57 am
Re: finding work with minimal information
You take energy absorbed and in that case q would negative because the system is doing work (energy lost). You use the delta U = q + w equation. And given delta U and q, you would find work
-
Lucas Vu 2G
- Posts: 87
- Joined: Fri Sep 29, 2023 12:25 pm
Re: finding work with minimal information
Using the equation 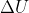 = q + w, you can solve for w as the
= q + w, you can solve for w as the 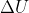 and the q are given to you as internal energy and energy absorbed by the surroundings.
and the q are given to you as internal energy and energy absorbed by the surroundings.
Re: finding work with minimal information
If you're dealing with a system where pressure-volume work is not the primary factor (for example, chemical reactions, phase changes), or if information about pressure and volume changes is not provided, then the formula W=q−ΔU can be used.
Re: finding work with minimal information
We can use the formula delta U = q + w and isolate for w (work) when given the change in internal energy energy absorbed by the experimental surroundings.
Return to “Calculating Work of Expansion”
Who is online
Users browsing this forum: No registered users and 6 guests

