Work Equations [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
SantanaRodriguezDis1G
- Posts: 56
- Joined: Sat Jul 22, 2017 3:00 am
Re: Work Equations
It depends in the situation. If the volume is constant, ΔV = 0 so w = 0. If the pressure is constant, w = -P(ext)ΔV. If you have an isothermal reversible expansion (both pressure and volume change by infinitely small amount), w = -nRTln(V2/V1). I hope that helps?
-
Natalie LeRaybaud 1G
- Posts: 54
- Joined: Thu Jul 13, 2017 3:00 am
Re: Work Equations
Generally it is based off the information given within the problem. For instance if the temperature and amount of moles or mass of substance is given with aa changing volume, it is probably best to use the 2nd equation. If you are given a constant value of pressure then it would be safe to use the first equation. But I have a feeling that we will be using the 2nd one more often in this chapter.
-
Erica Nagase 1H
- Posts: 30
- Joined: Sat Jul 22, 2017 3:00 am
Re: Work Equations
If the problem states it's irreversible, then we use 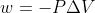 . If it's reversible, we use
. If it's reversible, we use 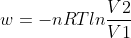 .
.
-
Jessica Nunez 1I
- Posts: 58
- Joined: Thu Jul 13, 2017 3:00 am
Re: Work Equations
As mentioned, if the work done is reversible, then you would use w = -nRTln(V2/V1). If the work done is irreversible, then you would use w = -P(ext)ΔV.
-
Alexia Joseph 2B
- Posts: 56
- Joined: Thu Jul 27, 2017 3:01 am
Re: Work Equations
Do we need to know how to derive these equations/the reasoning that's involved to come up with the equations ourselves?
-
Amelia Georgius 1K
- Posts: 25
- Joined: Fri Sep 29, 2017 7:05 am
- Been upvoted: 1 time
Re: Work Equations
You can use the equation w= -PdeltaV when pressure is constant
You can use the equation w= -nRt lnV2/V1 when temperature is constant
You can use the equation w= -nRt lnV2/V1 when temperature is constant
-
Shanmitha Arun 1L
- Posts: 53
- Joined: Fri Sep 29, 2017 7:04 am
- Been upvoted: 1 time
Re: Work Equations
It's easiest to choose equations based on the problem. You can tell by what they give what equation to use. Also, as mentioned, it depends on whether it is reversible or irreversible.
-
Magdalena Palavecino 1A
- Posts: 54
- Joined: Fri Sep 29, 2017 7:04 am
Re: Work Equations
Will the problem always say that the reaction is isothermal? Or will it not always be the case and how does this change your way of approaching the problem?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Work Equations [ENDORSED]
Problem could state the reactions occurs at 25 C.
But these type of questions involving work of expansion for a gas typically apply to simple systems (not reactions).
The only reaction they can apply to are gas phase reactions that result in different moles of gas (reactant) and gas (product).
But these type of questions involving work of expansion for a gas typically apply to simple systems (not reactions).
The only reaction they can apply to are gas phase reactions that result in different moles of gas (reactant) and gas (product).
Return to “Calculating Work of Expansion”
Who is online
Users browsing this forum: No registered users and 4 guests

