Confused about work formulas and specific heats
Moderators: Chem_Mod, Chem_Admin
-
Isa Samad 1L
- Posts: 53
- Joined: Fri Sep 29, 2017 7:07 am
- Been upvoted: 1 time
Confused about work formulas and specific heats
Was going through some of the homework question and was confused by the various formulas for work and when you can use them. For example, when should I use w=-P∆V vs w=-nRTln((V2/V1))? Also, are we only supposed to use Cv and Cp for ∆S calculations when temperature is changing? Can we use these specific heats for conditions in which temperature is constant but say pressure is changing (ie: ∆S = nRln((P1/P2)) or ∆S = nRCvln(P1/P2)?
-
Andres Reynoso 1J
- Posts: 30
- Joined: Fri Sep 29, 2017 7:06 am
Re: Confused about work formulas and specific heats
I'm pretty sure Cv and Cp are only to be used when the conditions dictate that the system is under constant volume or pressure, respectively, which is why it is often used when temperature is changing. For example, using Cp for a system where pressure is changing wouldn't work since pressure must be constant for it to work. As for when to use the different equations for work, I think that is circumstantial and depends on the information given and what you are trying to figure out. For instance, using 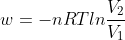 is done for calculating work done when an ideal gas expands reversibly and isothermally.
is done for calculating work done when an ideal gas expands reversibly and isothermally.
-
Peter Dis1G
- Posts: 97
- Joined: Fri Sep 29, 2017 7:04 am
Re: Confused about work formulas and specific heats
No, I don't think so. If the temperature is constant, there should be some calculation for the change in volume or pressure. Since the formulas in the book doesn't specify those conditions you said, there shouldn't be that condition to consider. Going over examples will help you develop a further understanding. Good luck!
Return to “Calculating Work of Expansion”
Who is online
Users browsing this forum: No registered users and 11 guests

