8.31 Calculate the heat released by 5.025 g of Kr(g) at
0.400 atm as it cools from 97.6 oC to 25.0 oC at (a) constant pressure and (b) constant volume. Assume that krypton behaves as an ideal gas.
I don't really know where to start with this problem or what equations to even use.
6th edition 8.31
Moderators: Chem_Mod, Chem_Admin
-
Matthew Tran 1H
- Posts: 165
- Joined: Fri Sep 28, 2018 12:16 am
Re: 6th edition 8.31
Treat this problem as you would any heat capacity problem. You should be using the relations 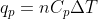 and
and 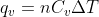 just as you would use the equation
just as you would use the equation 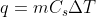 for ordinary heat capacity problems. You can find the equations for Cp and Cv on the equation sheet.
for ordinary heat capacity problems. You can find the equations for Cp and Cv on the equation sheet.
-
Nicolette_Canlian_2L
- Posts: 77
- Joined: Fri Sep 28, 2018 12:25 am
- Been upvoted: 1 time
Re: 6th edition 8.31
I was stuck on the same question. So we have to convert the given mass of the krypton gas into moles and work from there?
-
Catly Do 2E
- Posts: 64
- Joined: Fri Sep 28, 2018 12:24 am
Return to “Calculating Work of Expansion”
Who is online
Users browsing this forum: No registered users and 8 guests

