Can someone guide me through this problem?
"Suppose that 1.00 kJ of heat is transferred to 2.00 mol argon. What will the final temperature be if the heat is transferred at a constant pressure? Calculate the enthalpy change for the process and describe the thermodynamic reaction. Assume an ideal, monatomic gas at STP (25 Celcius, 1 atm)."
Any help is appreciated. Thanks!
Calculating final temp & enthalpy for an ideal monatomic gas
Moderators: Chem_Mod, Chem_Admin
-
Jia Hao Liu 3L
- Posts: 4
- Joined: Fri Sep 26, 2014 2:02 pm
-
Jenna Kovsky 1I
- Posts: 13
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Calculating final temp & enthalpy for an ideal monatomic
The first part (calculating the final temperature) is fairly straightforward. I used the equation q=n*C*deltaT. For the heat capacity (C), you were told to assume that it is an ideal, monatomic gas at constant pressure, so you would use 5/2 R as your heat capacity (this is given on the formula sheet). So you just plug in 5/2*8.314J/Kmol for C, 1.00kJ for q, 2.00mol for n, and 298K (because you're assuming STP) for T initial. Then you just solve for T final. Don't forget to convert q from kilojoules to joules (because the gas constant uses joules)
As for the part about calculating the enthalpy change, I'm still working on that personally, I'll post here again if I get it.
Hope that helped!
As for the part about calculating the enthalpy change, I'm still working on that personally, I'll post here again if I get it.
Hope that helped!
-
Jenna Kovsky 1I
- Posts: 13
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Calculating final temp & enthalpy for an ideal monatomic
Actually, now that I think about it, doesn't q=deltaH at constant pressure?
-
Jia Hao Liu 3L
- Posts: 4
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Calculating final temp & enthalpy for an ideal monatomic
Yeah, that's what i was thinking... So delta H = 1.00 kJ and the reaction is endothermic?
-
Jenna Kovsky 1I
- Posts: 13
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Calculating final temp & enthalpy for an ideal monatomic
That seems right to me! Where did you get this problem, maybe you can look up the solution online or something?
-
Jia Hao Liu 3L
- Posts: 4
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Calculating final temp & enthalpy for an ideal monatomic
It was on my quiz! Thanks for your help!
-
Jenna Kovsky 1I
- Posts: 13
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Calculating final temp & enthalpy for an ideal monatomic
That seems right to me! Where did you get this problem, maybe you can look up the solution online or something?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Calculating final temp & enthalpy for an ideal monatomic
Correct, 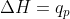 is true always. Since this process is indeed constant pressure, then
is true always. Since this process is indeed constant pressure, then 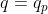
Return to “Calculating Work of Expansion”
Who is online
Users browsing this forum: No registered users and 4 guests

