Work without volume
Moderators: Chem_Mod, Chem_Admin
-
Abigail Menchaca_1H
- Posts: 104
- Joined: Sat Sep 07, 2019 12:19 am
-
TimVintsDis4L
- Posts: 104
- Joined: Sat Aug 17, 2019 12:17 am
Re: Work without volume
I don't know if this really helps, but if we are given the Internal Energy, q, or things that can be used to find q such as 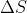 and t, then we can find q using
and t, then we can find q using 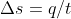 . Once you find the q, you can plug it into
. Once you find the q, you can plug it into 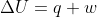 to get w.
to get w.
Re: Work without volume
The only types of work we need to know for thermodynamics are expansion work and non-expansion work, which involve volume change.
Re: Work without volume
since what we learned involves expansion of volume, we need the change in volume to see how much work is done
-
Chloe Alviz 1E
- Posts: 102
- Joined: Sat Aug 17, 2019 12:17 am
Re: Work without volume
I think most of the problems that we do involve expansion work (so a change in volume is given), but since you can relate work with U = q + w, I assume you can find it through this equation given the internal energy and enthalpy.
-
Ryan Yee 1J
- Posts: 101
- Joined: Sat Aug 17, 2019 12:16 am
Re: Work without volume
Since w=p(deltaV), not knowing the volume would mean you cant calculate work. But since U= q + w, given internal energy and enthalpy, you could calculate work.
Return to “Calculating Work of Expansion”
Who is online
Users browsing this forum: No registered users and 5 guests

