I understand that we use the equation w = -P(delta V) when referring to a gas that is at a constant pressure. However, I'm not sure if I am correct in assuming that we use the integral equation when the gas is NOT at a constant pressure.
So essentially my question is: how do we know when to use the integral equation versus the w = - P(delta V) equation?
Constant Pressure
Moderators: Chem_Mod, Chem_Admin
-
Elaine Steinberg 3H
- Posts: 105
- Joined: Fri Sep 24, 2021 6:47 am
Re: Constant Pressure
Hi!
The integral equation can also be used for changes in volume at constant pressure, which means you can take P out of the integral.
To determine when to use each, look at how small or large the change in volume is. Small changes in volume, when the system is in equilibrium with the surroundings, requires the sum of many small changes and thus requires the integral. For when larger more sudden changes occur, use P*deltaV.
The integral equation can also be used for changes in volume at constant pressure, which means you can take P out of the integral.
To determine when to use each, look at how small or large the change in volume is. Small changes in volume, when the system is in equilibrium with the surroundings, requires the sum of many small changes and thus requires the integral. For when larger more sudden changes occur, use P*deltaV.
Last edited by Elaine Steinberg 3H on Fri Jan 28, 2022 2:11 pm, edited 1 time in total.
-
Ashley Wilson 2L
- Posts: 104
- Joined: Fri Sep 24, 2021 5:24 am
Re: Constant Pressure
You are correct. Use the 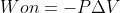 equation when the pressure is held constant. The
equation when the pressure is held constant. The 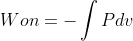 equation is more general and can be used to calculate the work on a gas when pressure is not constant.
equation is more general and can be used to calculate the work on a gas when pressure is not constant.
-
Mario Prado 1K
- Posts: 101
- Joined: Fri Sep 24, 2021 6:22 am
Re: Constant Pressure
Hello,
Yup you are right. You use the regular equation when pressure is constant and then when it isn't you use the integral equation.
Hope this helps.
Yup you are right. You use the regular equation when pressure is constant and then when it isn't you use the integral equation.
Hope this helps.
-
Reagan Feldman 1D
- Posts: 102
- Joined: Fri Sep 24, 2021 5:44 am
- Been upvoted: 2 times
Re: Constant Pressure
When the internal and external pressures of a system are about the same (system at equilibrium), that means that any changes in volume would be extremely small, so we use the integral equation to calculate for the many steps of the slow expansion. This is a reversible process. When the pressure is constant, changes in volume would lead to sudden expansion, so we get rid of the integral and use w=-PΔV. This is an irreversible process.
-
Caitlin_Doak_2H
- Posts: 52
- Joined: Wed Feb 17, 2021 12:18 am
-
Aaron Kwan 3B
- Posts: 101
- Joined: Fri Sep 24, 2021 6:07 am
Re: Constant Pressure
If you mean chemical equilibrium, not usually. In order to solve chemical equilibrium problems, the usual method is to construct an ICE table.
-
Kathryn Heinemeier 3H
- Posts: 104
- Joined: Fri Sep 24, 2021 5:09 am
Re: Constant Pressure
yes, when the pressure is constant we can use the w=-pdeltav; when it is not constant we would have to use the integral equation.
-
Warren Jolicoeur 1B
- Posts: 100
- Joined: Fri Sep 24, 2021 5:37 am
Re: Constant Pressure
Caitlin_Doak_2H wrote:Are integrals used in equilibrium ?
No, i dont belive so.
Return to “Calculating Work of Expansion”
Who is online
Users browsing this forum: No registered users and 5 guests

