I am a bit confused on number 14. It reads as follows:
Automobile airbags contain solid sodium azide, NaN3,
that reacts to produce nitrogen gas when heated, thus inflating the bag.
2NaN3(s)⟶2Na(s)+3N2(g)
Calculate the value of work, w, for the system if 18.4 g NaN3 reacts completely at 1.00 atm and 22∘ C.
Week 3/4 HW Problem #14
Moderators: Chem_Mod, Chem_Admin
-
ACabello 1G
- Posts: 10
- Joined: Mon Jan 09, 2023 2:48 am
Re: Week 3/4 HW Problem #14
Sorry if my explanation is confusing, but what you are looking for is work, so you use the equation 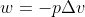 .
.
In the example, you are given Pressure, grams of NaN3, and temperature. So, since you are not given volume, you can use the equation PV=nRT to find it. Instead of using the moles of NaN3 though, since N2 is the actual gas in the equation, use stoichiometry to figure out the moles of N2. From there, plug in your values and find the volume. (Look at your units carefully).
Then after you find your volume, find your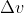 (Final - Initial) and find w. Don't forget to convert it to joules after.
(Final - Initial) and find w. Don't forget to convert it to joules after.
In the example, you are given Pressure, grams of NaN3, and temperature. So, since you are not given volume, you can use the equation PV=nRT to find it. Instead of using the moles of NaN3 though, since N2 is the actual gas in the equation, use stoichiometry to figure out the moles of N2. From there, plug in your values and find the volume. (Look at your units carefully).
Then after you find your volume, find your
-
ElizabethTopalian 2K
- Posts: 37
- Joined: Mon Jan 09, 2023 9:31 am
-
Emily Diep 2L
- Posts: 36
- Joined: Mon Jan 09, 2023 9:36 am
Re: Week 3/4 HW Problem #14
Hi!
I included my work below with steps to follow along! For Step 1, since we are given NaN3 in grams, use this to find the amount of moles of NaN3. Then, in Step 2, find the moles of N2 by multiplying the moles of NaN3 by (3/2) since there are 3N2 for every 2NaN3. Next, in Step 3, find the change in V by manipulating PV=nRT (this step is shown with blue highlight). Also, do not forget to change from C to K for temperature. Then, for Step 4, using
w=-Pex*deltaV, substitute the pressure given and the volume found in Step 3. Finally, in Step 5, convert to joules. I hope this helps!
I included my work below with steps to follow along! For Step 1, since we are given NaN3 in grams, use this to find the amount of moles of NaN3. Then, in Step 2, find the moles of N2 by multiplying the moles of NaN3 by (3/2) since there are 3N2 for every 2NaN3. Next, in Step 3, find the change in V by manipulating PV=nRT (this step is shown with blue highlight). Also, do not forget to change from C to K for temperature. Then, for Step 4, using
w=-Pex*deltaV, substitute the pressure given and the volume found in Step 3. Finally, in Step 5, convert to joules. I hope this helps!
-
Cate Cattano 1L
- Posts: 36
- Joined: Mon Jan 09, 2023 8:41 am
-
Grace Strottman 2B
- Posts: 47
- Joined: Mon Jan 09, 2023 8:51 am
Re: Week 3/4 HW Problem #14
Hi Cate,
The 0.082057 value is the constant R for the unit L*atm/(mol * K) which is are the units were working with. You can find this number in the chempendix
The 0.082057 value is the constant R for the unit L*atm/(mol * K) which is are the units were working with. You can find this number in the chempendix
Return to “Calculating Work of Expansion”
Who is online
Users browsing this forum: No registered users and 4 guests

