Copper (II) nitrate reacts with sodium hydroxide to produce a precipitate of light blue copper(II) hydroxide. (a) Write the net ionic equation for the reaction.
I am a bit rusty on this, so my question is, how would we go about writing the net ionic equation for this particular reaction, or any reaction, in general? Thank you!
Fundamentals M.9 [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Britney Pheng 1L
- Posts: 22
- Joined: Wed Sep 21, 2016 2:56 pm
- Been upvoted: 1 time
Re: Fundamentals M.9 [ENDORSED]
Hi Michelle!
What I do first is write out the complete equation:
_{2} (aq) + 2 NaOH (aq) \rightarrow Cu(OH)_{2} (s) + 2 NaNO_{3} (aq))
(We get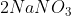 from the other products in the chemical reaction)
from the other products in the chemical reaction)
Next, we want to write out the complete ionic equation, which is just all the dissociated ions in the equation:+2 NO_{3}^{-} (aq) + 2 Na^{+} (aq) + 2OH^{-} (aq) \rightarrow Cu(OH)_{2} (s) + 2 Na^{+} (aq) + 2NO_{3}^{-} (aq))
(_{2}) is not changed because it is insoluble. This is where solubility rules come into play, which lets you know the ionic compounds that will not dissociate)
is not changed because it is insoluble. This is where solubility rules come into play, which lets you know the ionic compounds that will not dissociate)
Then, we can get our net ionic equation by cancelling out the ions that are on both sides of the reaction arrow
(These are called spectator ions and they do not participate in the reaction)
When simplified, we get the net ionic equation:
 + 2OH^{-} (aq) \rightarrow Cu(OH)_{2} (s))
So as a quick summary:
- Write out the complete molecular equation
- Write out the complete ionic equation by separating the compounds (using solubility rules)
- Cancel out the spectator ions on both sides to get the net ionic equation
Hope that helps!
What I do first is write out the complete equation:
(We get
Next, we want to write out the complete ionic equation, which is just all the dissociated ions in the equation:
(
Then, we can get our net ionic equation by cancelling out the ions that are on both sides of the reaction arrow
(These are called spectator ions and they do not participate in the reaction)
When simplified, we get the net ionic equation:
So as a quick summary:
- Write out the complete molecular equation
- Write out the complete ionic equation by separating the compounds (using solubility rules)
- Cancel out the spectator ions on both sides to get the net ionic equation
Hope that helps!
-
Parsia Vazirnia 2L
- Posts: 44
- Joined: Fri Jul 22, 2016 3:00 am
Re: Fundamentals M.9
Hi,
Where does the Na(N03) come from in the products side of the equation? The question only mentions that it produces a precipitate of light blue copper(II) hydroxide.I know there must be a sodium in products side because we have one in the reactants, but how do you know its Na(N03) because it is not stated in the question?
Where does the Na(N03) come from in the products side of the equation? The question only mentions that it produces a precipitate of light blue copper(II) hydroxide.I know there must be a sodium in products side because we have one in the reactants, but how do you know its Na(N03) because it is not stated in the question?
-
Britney Pheng 1L
- Posts: 22
- Joined: Wed Sep 21, 2016 2:56 pm
- Been upvoted: 1 time
Re: Fundamentals M.9
Hi Parsia,
Sorry for the late reply! In this question, you are given two compounds that react together to form two new compounds.
This is called a double replacement/displacement reaction: AB + XY XB + AY
XB + AY
The products are formed when the two cations (or two anions) of the reactants switch places.
We are given_{2}) (
(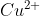 &
& 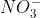 ) and
) and  (
(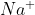 &
& 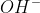 ).
).
So when we switch the cations of the reactants (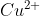 and
and 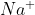 ), you will get both: the stated precipitate
), you will get both: the stated precipitate _{2}) and the inferred compound
and the inferred compound 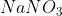 .
.
Even though it’s not stated, we can’t leave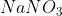 out because that would violate the law of conservation of mass.
out because that would violate the law of conservation of mass.
Hope this clears it up.
Sorry for the late reply! In this question, you are given two compounds that react together to form two new compounds.
This is called a double replacement/displacement reaction: AB + XY
The products are formed when the two cations (or two anions) of the reactants switch places.
We are given
So when we switch the cations of the reactants (
Even though it’s not stated, we can’t leave
Hope this clears it up.
Return to “Limiting Reactant Calculations”
Who is online
Users browsing this forum: No registered users and 8 guests

