When to use H vs H2
Moderators: Chem_Mod, Chem_Admin
-
Aidan Ryan 1B
- Posts: 60
- Joined: Fri Sep 28, 2018 12:17 am
When to use H vs H2
One thing I am struggling to figure out is if I have 1 mol of Hydrogen gas (H2) is that the same (in terms of atoms of Hydrogen) as having 1 mol of Hydrochloric Acid (HCL)? Its been on my mind and on some questions I struggled to know which molar mass to use (as in 1.008 or double that) when given H2 has a reactant.
-
Lydia Luong 4L
- Posts: 59
- Joined: Fri Sep 28, 2018 12:24 am
-
Jessica Dharmawan 1G
- Posts: 32
- Joined: Fri Sep 28, 2018 12:15 am
Re: When to use H vs H2
I believe you do multiply by 2 when using H2. Because there are two atoms of hydrogen, you would multiply the molar mass of 1 H atom by 2 to get the total molar mass of H2.
-
Saima Salam 3J
- Posts: 32
- Joined: Fri Sep 28, 2018 12:25 am
Re: When to use H vs H2
When you have 1 mole of H2, the molar mass will be the molar mass of Hydrogen times 2 because you have 2 atoms of hydrogen in this molecule. However, in HCl, you will only use 1.007 because there is only one atom of Hydrogen. The subscript next to the Hydrogen will indicate how much of that atom there is so if there is no subscript, for example, in HCl, then just use the regular Hydrogen molar mass.
-
Sydney To 1D
- Posts: 60
- Joined: Fri Sep 28, 2018 12:15 am
Re: When to use H vs H2
In the case in which 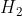 is presented as a diatomic molecule, the molar mass of H would be multiplied by two. Therefore, the molar mass of
is presented as a diatomic molecule, the molar mass of H would be multiplied by two. Therefore, the molar mass of 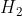 is 2.016
is 2.016 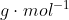 .
.
-
Adriana_4F
- Posts: 74
- Joined: Fri Sep 28, 2018 12:29 am
Re: When to use H vs H2
I also have a question: when you have a reaction in the form of MgSO4.7H2O where the 7 is in front of the H2O, would you have to do 7x(molar mass of H2) + the molar mass of O or 7x(the molar mass of H20)?
Re: When to use H vs H2
I remember in high school that there were some elements that always had to be in pairs. The reasoning was that hydrogen would be unstable as a singular atom in itself. So I guess it is necessary to have to do an extra step of multiplying molar mass by 2.
-
Dhwani Krishnan 1G
- Posts: 63
- Joined: Fri Sep 28, 2018 12:17 am
Re: When to use H vs H2
Adriana_4H wrote:I also have a question: when you have a reaction in the form of MgSO4.7H2O where the 7 is in front of the H2O, would you have to do 7x(molar mass of H2) + the molar mass of O or 7x(the molar mass of H20)?
Because 2 hydrogens are Bonded to 1 oxygen (to form water), the "7" is the coefficient for the entire water molecule (H2O), not just the H2. Therefore, you do 7x(molar mass of H2O).
Return to “Limiting Reactant Calculations”
Who is online
Users browsing this forum: No registered users and 8 guests

