Solve this exercise without using a calculator. The reaction 6 ClO 2 (g) + 2 BrF 3 (l) → 6 ClO 2 F (s) + Br 2 (l) is carried out with 12 mol ClO 2 and 5 mol BrF 3 . (a) Identify the excess reactant. (b) Estimate how many moles of each product will be produced and how many moles of the excess reactant will remain.
I calculated that the excess reactant is BrF since there is 4 mol needed while CLO2 needs 15 mol. However, I do not understand how to estimate how many moles of each product will be produced because if there are 15 moles needed for the reaction of CLO2, how can a reaction occur if we are only given 12 mol CLO2?
Textbook M5
Moderators: Chem_Mod, Chem_Admin
-
Anh Trinh 1J
- Posts: 156
- Joined: Wed Sep 30, 2020 9:55 pm
- Been upvoted: 1 time
Re: Textbook M5
a) You are right that 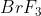 is the excess reactant.
is the excess reactant.
b) To calculate how many moles of each product will be produced, you have to find how many moles of each reactants are required for each time it is reacted. In the chemical reaction, 6 moles of and 1 mole of
and 1 mole of 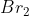 are produced from 6 moles
are produced from 6 moles  and 2 moles
and 2 moles 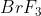 . Because
. Because  is the limiting reactant, we will use this to find how many moles of each product will be produced. We have 12 mol
is the limiting reactant, we will use this to find how many moles of each product will be produced. We have 12 mol  , and there is 6 required to produce 1
, and there is 6 required to produce 1 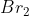 , then 2 mol
, then 2 mol 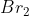 is produced. 6 mole
is produced. 6 mole  is required to produce 6
is required to produce 6  , and since we have 12 mol
, and since we have 12 mol  , 12 mol
, 12 mol  will be produced.
will be produced.
To find how many moles of the excess reactant (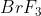 ) will remain, we have to note that 2 mol
) will remain, we have to note that 2 mol 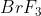 are used for every 6 mol
are used for every 6 mol  , and because we have 12 mol
, and because we have 12 mol  (the limiting reactant), 4 mol (
(the limiting reactant), 4 mol (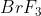 ) are required. We were given 5 mol (
) are required. We were given 5 mol (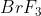 ) and only used 4, so there is 1 mol (
) and only used 4, so there is 1 mol (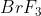 ) remaining.
) remaining.
b) To calculate how many moles of each product will be produced, you have to find how many moles of each reactants are required for each time it is reacted. In the chemical reaction, 6 moles of
To find how many moles of the excess reactant (
-
Sedge Greenlee
- Posts: 90
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Textbook M5
Hey! Anh Trinh gave a great explanation for this exact problem. For a more general explanation for solving limiting reaction problems, keep in mind that the chemical formula in terms of moles can be thought of as a ratio. For example, in this problem, for even 6 moles of ClO2, we produce 1 mole of Br2 and 6 moles of ClO2F. with this relationship established between the limiting reactant and the products, we can calculate how many moles of product are produced in relation to how many moles of the limiting reactant is provided. 2 sets of 6 moles of ClO2 produce 2 sets of 1 mole of Br2 and 2 sets of 6 moles of ClO2F, AKA 2 moles of Br2 and 12 moles of ClO2F.
Return to “Limiting Reactant Calculations”
Who is online
Users browsing this forum: No registered users and 6 guests

