Aluminum metal reacts with chlorine gas to produce aluminum chloride. In one preparation, 255 g of aluminum is placed in a container holding 535 g of chlorine gas. After reaction ceases, it is found that 300. g of aluminum chloride has been produced. (a) Write the balanced equation for the reaction. (b) What mass of aluminum chloride can be produced by these reactants? (c) What is the percentage yield of aluminum chloride?
Can someone help me with identifying the limiting reactant? I balanced the equation to 2Al + 3Cl2 --> 2AlCl3 and found the moles to be 9.45 mol Al and 7.545 mol Cl2. I know the ratio is 2 mol Al per 3 mol Cl2 but how would I find out which is the limiting factor without just using common sense and seeing Cl2 is not enough (if that makes sense)... like when the ratio is 1:2, I would multiply the first number by 2 and see if there is enough but with this one, I'm not sure what to do.
Textbook Problem M15
Moderators: Chem_Mod, Chem_Admin
-
SuryaDham 3E
- Posts: 100
- Joined: Fri Sep 24, 2021 7:29 am
Re: Textbook Problem M15
Hey le, I think first off you accidentally used 225 g of Al instead of the given 255
The actual molar amount for Al would be 9.45
to find the limiting reactant, i would multiply these molar amounts by the ratio of the moles of AlCl3: the ratio of the reactants
For example, the way I would do it would be to multiply 9.45 mol Al by the ratio of 2 mol AlCl3: 2 mol Al
Then, i would multiply 7.545 mol Cl2 by the ratio 2 mol AlCl3: 3 mol Cl2
Once you do these multiplications, the lower molar value will be your limiting reactant.
Hope this helps
The actual molar amount for Al would be 9.45
to find the limiting reactant, i would multiply these molar amounts by the ratio of the moles of AlCl3: the ratio of the reactants
For example, the way I would do it would be to multiply 9.45 mol Al by the ratio of 2 mol AlCl3: 2 mol Al
Then, i would multiply 7.545 mol Cl2 by the ratio 2 mol AlCl3: 3 mol Cl2
Once you do these multiplications, the lower molar value will be your limiting reactant.
Hope this helps
-
Vincent Nguyen 3G
- Posts: 104
- Joined: Fri Sep 24, 2021 6:00 am
- Been upvoted: 1 time
Re: Textbook Problem M15
YMMV, but this is my approach. Also, I believe you may have used the wrong mass of Al when calculating the moles, since I got 9.451 moles of Al and it looks like Surya did as well. Knowing that we use up 2 mol of Al for each 3 mol of Cl_2 every time that we run this equation, you can divide your amount of mol of Al (9.451) by 2 and then your amount of mol of Cl_2 (7.545) by 3 to get an idea of how many times you could "run this equation." So, 9.451/2 means that your amount of Al allows you to run this equation 4.725 times while 7.545/3 means that you amount of Cl_2 allows you to run this equation 2.515 times, thus Cl_2 is the limiting reactant since it allows fewer "runs." Let me know if this helps, cheers.
-
Ryan Khiev 1L
- Posts: 109
- Joined: Fri Sep 24, 2021 5:44 am
- Been upvoted: 1 time
Re: Textbook Problem M15
Hi Vy,
A foolproof way to find the limiting reactant is to convert the reactants into product using the moles from each reactant.
For example, you already got the moles of Al(9.45) and Cl2(7.55). Now, you would convert these moles into moles of reactant using the molar ratio from the balanced equation:
9.45 mol Al * (2 mol AlCl3/2 mol Al)= 9.45 mol AlCl3
7.55 mol Cl2 * (2 mol AlCl3/3mol Cl2) = 5.03 mol AlCl3
Since 5.03<9.45, Cl2 is the limiting reactant, and to solve the rest of part b, you would need to convert 5.03 mol AlCl3 into grams using the molar mass of AlCl3.
Hope this helps!
A foolproof way to find the limiting reactant is to convert the reactants into product using the moles from each reactant.
For example, you already got the moles of Al(9.45) and Cl2(7.55). Now, you would convert these moles into moles of reactant using the molar ratio from the balanced equation:
9.45 mol Al * (2 mol AlCl3/2 mol Al)= 9.45 mol AlCl3
7.55 mol Cl2 * (2 mol AlCl3/3mol Cl2) = 5.03 mol AlCl3
Since 5.03<9.45, Cl2 is the limiting reactant, and to solve the rest of part b, you would need to convert 5.03 mol AlCl3 into grams using the molar mass of AlCl3.
Hope this helps!
-
Maxwell Yao
- Posts: 102
- Joined: Fri Sep 24, 2021 5:38 am
Re: Textbook Problem M15
The limiting reactant is the reactant that gets all used up. In this case, we do not know whether Al or Cl2 is the reactant that is limiting. Therefore we have to use dimensional analysis to see how much of the product (AlCl3) can be theoretically be made by 255g of Al or 535g of Cl2.
To test 255g of Al, we find the moles of Al and then use the mole ratios to find out how many moles of AlCl3 that would produce, and then we convert that to grams
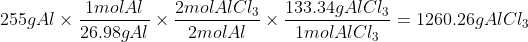
To test 535g of Cl2, we do the same process as above.
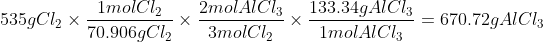
As you can see from above, Cl2 is clearly the limiting reagent because it produces less of the product (AlCl3).
Hope this helps!
To test 255g of Al, we find the moles of Al and then use the mole ratios to find out how many moles of AlCl3 that would produce, and then we convert that to grams
To test 535g of Cl2, we do the same process as above.
As you can see from above, Cl2 is clearly the limiting reagent because it produces less of the product (AlCl3).
Hope this helps!
Return to “Limiting Reactant Calculations”
Who is online
Users browsing this forum: No registered users and 7 guests

