Achieve Week 1 Homework, Question 10
Moderators: Chem_Mod, Chem_Admin
-
maliamichelsen_3a
- Posts: 98
- Joined: Fri Sep 24, 2021 5:20 am
Achieve Week 1 Homework, Question 10
Hi everyone,
I'm having some trouble understanding the steps needed to get through this problem. I was able to figure out the molecular formulas for the reaction shown in the problem and convert the given quantities of 2-butanone and 3‑methyl‑3‑hexanol to moles, but I'm not entirely sure where to go from there. If anyone could also explain to me the difference between theoretical and actual yields that would be fantastic as well since I seem to be getting these confused a lot.
Here is the problem (without the diagram of the reaction) for quick reference:
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made in situ by reacting 1‑bromopropane with metallic magnesium, to make 3‑methyl‑3‑hexanol. A reaction was performed in which 0.25 mL of 2‑butanone was reacted with an excess of propyl magnesiumbromide to make 0.281 g of 3‑methyl‑3‑hexanol. Calculate the theoretical yield and percent yield for this reaction.
I'm having some trouble understanding the steps needed to get through this problem. I was able to figure out the molecular formulas for the reaction shown in the problem and convert the given quantities of 2-butanone and 3‑methyl‑3‑hexanol to moles, but I'm not entirely sure where to go from there. If anyone could also explain to me the difference between theoretical and actual yields that would be fantastic as well since I seem to be getting these confused a lot.
Here is the problem (without the diagram of the reaction) for quick reference:
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made in situ by reacting 1‑bromopropane with metallic magnesium, to make 3‑methyl‑3‑hexanol. A reaction was performed in which 0.25 mL of 2‑butanone was reacted with an excess of propyl magnesiumbromide to make 0.281 g of 3‑methyl‑3‑hexanol. Calculate the theoretical yield and percent yield for this reaction.
-
Nathan Tran 1B
- Posts: 104
- Joined: Fri Sep 24, 2021 6:21 am
- Been upvoted: 1 time
Re: Achieve Week 1 Homework, Question 10
Hi, so first you would find the mass of 2-butanone which can be done because you are given the volume(.25ml) and the density(.81g/ml). To find the mass of of 2-butanone, use the equation density=mass/volume or .81g/ml=mass/.25ml and solve for mass. Then convert the mass of butanone into moles using its molar mass. After, you can multiple by the molar ratios but in this case you would be multipling by 1mol of 3-methyl-3-hexanone/1 mole of 2-butanone because it is a 1:1 ratio. This will give you moles of 3-methyl-3-hexanone. After finding moles of 3-methyl-3-hexanone you can then multiple by its molar mass to find the grams of 3-methyl-3-hexanone that should be created in this reaction.
The theoretical yield is the amount of product that should be produced through calculation while the actual yield is the amount of product that was actually produced in the experiment. Thus the calculations above provides you the theoretical yield. In the problem, they also offer the amount of actual product was produced(.281g) which is thus the actual yield.
Percent yield = 0.281 g/theoretical yield of 3-methyl-3-hexanone x 100%
The theoretical yield is the amount of product that should be produced through calculation while the actual yield is the amount of product that was actually produced in the experiment. Thus the calculations above provides you the theoretical yield. In the problem, they also offer the amount of actual product was produced(.281g) which is thus the actual yield.
Percent yield = 0.281 g/theoretical yield of 3-methyl-3-hexanone x 100%
-
Julie Mai 1K
- Posts: 100
- Joined: Fri Sep 24, 2021 6:46 am
Re: Achieve Week 1 Homework, Question 10
Hi!
Step 1: Find the mass of the product 3-methyl-3-hexanol by multiplying the 0.25mL of 2-butanone by its density of 0.81 g/mL. Since 2-butanone is reacting with excess propyl magnesiumbromide, 2-butanone would be the limiting reactant.
Step 2: Multiply it by 2-butanone's molar mass. To find the molar mass, you look at the diagram and at each point there should be an implied Carbon. Carbon always has four bonds so the rest of the bonds that are not shown should be an implied Hydrogen. Therefore, 2-butanone's chemical compound is C4H8O and the molar mass is 72.11g.
Step 3: Multiply by the molar ratio of 1 mol 2-butanone/1 mol product.
Step 4: Multiply that by the molar mass of the product. You can find the molar mass of the product by following step 2.
Step 5: Once you have the grams of the product, that will be your theoretical yield. To find percent yield, divide the actual yield given in the problem by the theoretical yield and multiply by 100.
Theoretical yield is the amount of product that should be produced in the reaction. Actual yield is the amount of product that's actually produced. During a reaction, there are usually side reactions or impurities, the actual yield is usually less than the theoretical yield.
Step 1: Find the mass of the product 3-methyl-3-hexanol by multiplying the 0.25mL of 2-butanone by its density of 0.81 g/mL. Since 2-butanone is reacting with excess propyl magnesiumbromide, 2-butanone would be the limiting reactant.
Step 2: Multiply it by 2-butanone's molar mass. To find the molar mass, you look at the diagram and at each point there should be an implied Carbon. Carbon always has four bonds so the rest of the bonds that are not shown should be an implied Hydrogen. Therefore, 2-butanone's chemical compound is C4H8O and the molar mass is 72.11g.
Step 3: Multiply by the molar ratio of 1 mol 2-butanone/1 mol product.
Step 4: Multiply that by the molar mass of the product. You can find the molar mass of the product by following step 2.
Step 5: Once you have the grams of the product, that will be your theoretical yield. To find percent yield, divide the actual yield given in the problem by the theoretical yield and multiply by 100.
Theoretical yield is the amount of product that should be produced in the reaction. Actual yield is the amount of product that's actually produced. During a reaction, there are usually side reactions or impurities, the actual yield is usually less than the theoretical yield.
-
Calvin Su 1B
- Posts: 50
- Joined: Fri Sep 24, 2021 6:06 am
Re: Achieve Week 1 Homework, Question 10
Hi Malia,
My process was pretty much the same as those who responded before me, so I'll just help reiterate the difference between theoretical and actual yield.
The theoretical yield is a (hypothetical) measurement of the maximum amount of product from a reaction under ideal conditions. The actual yield is the sum of product from a reaction that has actually taken place. The actual yield should (in theory) be less than the theoretical yield. The percent yield is then just the actual yield divided by the theoretical yield, multiplied by one hundred. py= (ay/ty) x 100
My process was pretty much the same as those who responded before me, so I'll just help reiterate the difference between theoretical and actual yield.
The theoretical yield is a (hypothetical) measurement of the maximum amount of product from a reaction under ideal conditions. The actual yield is the sum of product from a reaction that has actually taken place. The actual yield should (in theory) be less than the theoretical yield. The percent yield is then just the actual yield divided by the theoretical yield, multiplied by one hundred. py= (ay/ty) x 100
-
trishaferrer
- Posts: 92
- Joined: Fri Sep 24, 2021 5:26 am
Re: Achieve Week 1 Homework, Question 10
To find the theoretical yield, I multiplied the mass of 2-butanone to the molar mass of the product and divided it by the molar mass of 2-butanone. Since the actual yield was given, I used the equation, plugged in both values, and multiple by 100% to find the percent yield.
Re: Achieve Week 1 Homework, Question 10
maliamichelsen_3a wrote:Hi everyone,
I'm having some trouble understanding the steps needed to get through this problem. I was able to figure out the molecular formulas for the reaction shown in the problem and convert the given quantities of 2-butanone and 3‑methyl‑3‑hexanol to moles, but I'm not entirely sure where to go from there. If anyone could also explain to me the difference between theoretical and actual yields that would be fantastic as well since I seem to be getting these confused a lot.
Here is the problem (without the diagram of the reaction) for quick reference:
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made in situ by reacting 1‑bromopropane with metallic magnesium, to make 3‑methyl‑3‑hexanol. A reaction was performed in which 0.25 mL of 2‑butanone was reacted with an excess of propyl magnesiumbromide to make 0.281 g of 3‑methyl‑3‑hexanol. Calculate the theoretical yield and percent yield for this reaction.
I kept messing up this problem just because I was using an incorrect molar mass. Just wanted to remind everyone to double check your molar masses if you are struggling with this problem!
-
Ainsley McCabe 2D
- Posts: 101
- Joined: Fri Sep 24, 2021 6:54 am
Re: Achieve Week 1 Homework, Question 10
705573518 wrote:maliamichelsen_3a wrote:Hi everyone,
I'm having some trouble understanding the steps needed to get through this problem. I was able to figure out the molecular formulas for the reaction shown in the problem and convert the given quantities of 2-butanone and 3‑methyl‑3‑hexanol to moles, but I'm not entirely sure where to go from there. If anyone could also explain to me the difference between theoretical and actual yields that would be fantastic as well since I seem to be getting these confused a lot.
Here is the problem (without the diagram of the reaction) for quick reference:
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made in situ by reacting 1‑bromopropane with metallic magnesium, to make 3‑methyl‑3‑hexanol. A reaction was performed in which 0.25 mL of 2‑butanone was reacted with an excess of propyl magnesiumbromide to make 0.281 g of 3‑methyl‑3‑hexanol. Calculate the theoretical yield and percent yield for this reaction.
I kept messing up this problem just because I was using an incorrect molar mass. Just wanted to remind everyone to double check your molar masses if you are struggling with this problem!
Yes! I agree, I also had to redo this problem multiple times because my math was off so make sure to double check the simple math.
-
Ainsley McCabe 2D
- Posts: 101
- Joined: Fri Sep 24, 2021 6:54 am
Re: Achieve Week 1 Homework, Question 10
maliamichelsen_3a wrote:Hi everyone,
I'm having some trouble understanding the steps needed to get through this problem. I was able to figure out the molecular formulas for the reaction shown in the problem and convert the given quantities of 2-butanone and 3‑methyl‑3‑hexanol to moles, but I'm not entirely sure where to go from there. If anyone could also explain to me the difference between theoretical and actual yields that would be fantastic as well since I seem to be getting these confused a lot.
Here is the problem (without the diagram of the reaction) for quick reference:
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made in situ by reacting 1‑bromopropane with metallic magnesium, to make 3‑methyl‑3‑hexanol. A reaction was performed in which 0.25 mL of 2‑butanone was reacted with an excess of propyl magnesiumbromide to make 0.281 g of 3‑methyl‑3‑hexanol. Calculate the theoretical yield and percent yield for this reaction.
Another note I want to make is that I was told by one of the UAs that this problem is a little out of the scope of our class. So anyone that is extremely confused or worried because of the wording of this problem, don't! As long as you understand how to solve it you will be good!
-
Jamie Park 2F
- Posts: 59
- Joined: Wed Nov 25, 2020 12:19 am
Re: Achieve Week 1 Homework, Question 10
I just wanted to add that we start off our calculations with 2-butanone because the problem says that the propyl magnesiumbromide is in excess. 2-butanone will be the limiting reactant.
Also, the problem does not give us the chemical reaction formula, so I think we need to come up with our own formula from the picture (each line is a carbon atom, and hydrogens that are bonded to carbon are not shown in the drawings). However, we did not go over this yet, so I wouldn't worry too much about knowing this yet. I found the molar masses of 3-butanone and 3-methyl-3-hexanone online. They are 72.11g and 116.2g, respectively.
Also, the problem does not give us the chemical reaction formula, so I think we need to come up with our own formula from the picture (each line is a carbon atom, and hydrogens that are bonded to carbon are not shown in the drawings). However, we did not go over this yet, so I wouldn't worry too much about knowing this yet. I found the molar masses of 3-butanone and 3-methyl-3-hexanone online. They are 72.11g and 116.2g, respectively.
Re: Achieve Week 1 Homework, Question 10
Thanks everyone for all of your help! I am wondering, how did we know the molar ratio was 1:1? It was unclear to me from the question that we could assume there was one mole of 3-methyl-3-hexanol for every one mole of 2-butanone.
-
Aida Fraser 2I
- Posts: 61
- Joined: Fri Sep 24, 2021 5:32 am
Re: Achieve Week 1 Homework, Question 10
How do you find the molar mass of 2-butanone? I'm confused and having trouble using the diagram given
-
Aida Fraser 2I
- Posts: 61
- Joined: Fri Sep 24, 2021 5:32 am
Re: Achieve Week 1 Homework, Question 10
305607822 wrote:Thanks everyone for all of your help! I am wondering, how did we know the molar ratio was 1:1? It was unclear to me from the question that we could assume there was one mole of 3-methyl-3-hexanol for every one mole of 2-butanone.
Re: Achieve Week 1 Homework, Question 10
Hi guys, I was wondering if anyone could tell me how to find the actual yield given by the problem?
Re: Achieve Week 1 Homework, Question 10
Aida Fraser 2I wrote:How do you find the molar mass of 2-butanone? I'm confused and having trouble using the diagram given
Someone above said they looked online, which is what i did too. I think someone said there's a way to find it yourself, but we haven't covered that yet.
Re: Achieve Week 1 Homework, Question 10
Ainsley McCabe 2E wrote:705573518 wrote:maliamichelsen_3a wrote:Hi everyone,
I'm having some trouble understanding the steps needed to get through this problem. I was able to figure out the molecular formulas for the reaction shown in the problem and convert the given quantities of 2-butanone and 3‑methyl‑3‑hexanol to moles, but I'm not entirely sure where to go from there. If anyone could also explain to me the difference between theoretical and actual yields that would be fantastic as well since I seem to be getting these confused a lot.
Here is the problem (without the diagram of the reaction) for quick reference:
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made in situ by reacting 1‑bromopropane with metallic magnesium, to make 3‑methyl‑3‑hexanol. A reaction was performed in which 0.25 mL of 2‑butanone was reacted with an excess of propyl magnesiumbromide to make 0.281 g of 3‑methyl‑3‑hexanol. Calculate the theoretical yield and percent yield for this reaction.
I kept messing up this problem just because I was using an incorrect molar mass. Just wanted to remind everyone to double check your molar masses if you are struggling with this problem!
Yes! I agree, I also had to redo this problem multiple times because my math was off so make sure to double check the simple math.
Yes make sure to check your rounding on the theoretical yield. I had the right percent yield and the wrong theoretical yield, and it took me so long to realize it was because I just rounded incorrectly.
-
Ellen Brock 2I
- Posts: 100
- Joined: Fri Sep 24, 2021 5:34 am
Re: Achieve Week 1 Homework, Question 10
Abby 3G wrote:Aida Fraser 2I wrote:How do you find the molar mass of 2-butanone? I'm confused and having trouble using the diagram given
Someone above said they looked online, which is what i did too. I think someone said there's a way to find it yourself, but we haven't covered that yet.
I looked it up because there was no exact formula. I guess you could look up the formula and then calculate it from there but I just went straights to the number instead.
-
Ben Broselle 3 I
- Posts: 23
- Joined: Fri Sep 24, 2021 6:11 am
Re: Achieve Week 1 Homework, Question 10
couldnt figure out this question for the life of me. Figured out i was using the wrong molar mass. Had to take a 0 on the question though because i didnt look here first
-
Maggie Black 1C
- Posts: 101
- Joined: Fri Sep 24, 2021 7:21 am
Re: Achieve Week 1 Homework, Question 10
Theoretical yield is what you would get from a chemical reaction under "perfect" conditions, in the absence of limiting reactants, chemical disturbances, and other factors. Essentially, if the chemical reaction took place in a vacuum, then you would get the theoretical yield. However, the actual yield takes into account these factors (mostly the limiting reactants), so we calculate the actual yield using the amount of reactants we have available. Then we are able to compare these two numbers and calculate a percent yield (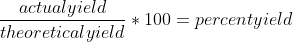
Re: Achieve Week 1 Homework, Question 10
I keep re-trying this problem and getting the wrong answers. Is 114.19 the correct molar mass of 3‑methyl‑3‑hexano?
Re: Achieve Week 1 Homework, Question 10
I realized that I was using the incorrect volume of 2-butanone. Thank you guys for your responses they really helped me!!
-
Diana peng 3I
- Posts: 31
- Joined: Thu Sep 30, 2021 5:05 am
Re: Achieve Week 1 Homework, Question 10
205282258 wrote:I keep re-trying this problem and getting the wrong answers. Is 114.19 the correct molar mass of 3‑methyl‑3‑hexano?
Hello, I think the molar mass of 3‑methyl‑3‑hexanone is 116.2. I got mine answer using 116.2. hope that helps
-
Dev Patel 1H
- Posts: 11
- Joined: Fri Sep 24, 2021 6:43 am
Re: Achieve Week 1 Homework, Question 10
Abby 3G wrote:Hi guys, I was wondering if anyone could tell me how to find the actual yield given by the problem?
You can't really find the actual yield without being given it, so you always have to be given the actual yield in order calculate the percent yield.
-
Dev Patel 1H
- Posts: 11
- Joined: Fri Sep 24, 2021 6:43 am
Re: Achieve Week 1 Homework, Question 10
205282258 wrote:I keep re-trying this problem and getting the wrong answers. Is 114.19 the correct molar mass of 3‑methyl‑3‑hexano?
No, the molecular weight for 3-methyl-3-hexanol is 116.20 g/mol.
-
Nicola Higgins 14B
- Posts: 103
- Joined: Fri Sep 24, 2021 5:41 am
Re: Achieve Week 1 Homework, Question 10
Nathan Tran 1B wrote: After, you can multiple by the molar ratios but in this case you would be multipling by 1mol of 3-methyl-3-hexanone/1 mole of 2-butanone because it is a 1:1 ratio.
Hi! How do you know there is a 1:1 ratio, and therefore no need to multiply the mol?
-
maliamichelsen_3a
- Posts: 98
- Joined: Fri Sep 24, 2021 5:20 am
Re: Achieve Week 1 Homework, Question 10
You guys are so helpful! I was able to figure everything out and I just wanted to say thanks :-)
Return to “Limiting Reactant Calculations”
Who is online
Users browsing this forum: No registered users and 7 guests

