Internal Energy
Moderators: Chem_Mod, Chem_Admin
-
Ashley Kenney 1E
- Posts: 32
- Joined: Wed Nov 14, 2018 12:23 am
Internal Energy
The textbook states that the internal energy of a monatomic ideal gas at a constant temperature is 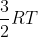 . How is this expression derived?
. How is this expression derived?
-
MaanasO 1A
- Posts: 72
- Joined: Fri Sep 28, 2018 12:26 am
Re: Internal Energy
Hi Ashley!
This equation relates to the kinetic energy of gas molecules. The derivation is mathematically rigorous, but you can check out the derivation here: http://hyperphysics.phy-astr.gsu.edu/hb ... ke.html#c1
Hope that helps!
This equation relates to the kinetic energy of gas molecules. The derivation is mathematically rigorous, but you can check out the derivation here: http://hyperphysics.phy-astr.gsu.edu/hb ... ke.html#c1
Hope that helps!
-
Jordan Lo 2A
- Posts: 85
- Joined: Fri Sep 28, 2018 12:25 am
-
Lisa Werner 2F
- Posts: 72
- Joined: Fri Sep 28, 2018 12:17 am
Re: Internal Energy
Dr. Lavelle explained it in lecture and it is required for several homework problems, so I think he considers it fair game for the midterm.
Return to “Concepts & Calculations Using First Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 5 guests

