[tex]\Delta U = \Delta H[/tex]
Moderators: Chem_Mod, Chem_Admin
-
David Sarkissian 1K
- Posts: 29
- Joined: Fri Sep 28, 2018 12:21 am
- Been upvoted: 1 time
[tex]\Delta U = \Delta H[/tex]
I'm just a little confused when you're suppose to disregard that 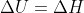 and instead use the equation
and instead use the equation 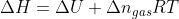 instead? What are the conditions that need to be met?
instead? What are the conditions that need to be met?
-
Ashley Zhu 1A
- Posts: 69
- Joined: Fri Sep 28, 2018 12:16 am
Re: [tex]\Delta U = \Delta H[/tex]
The two equations you have there are essentially the same equations derived from delta(U) = delta(H) + w where w is work. Since w = -Pdelta(V) where P is pressure and delta V is the change in volume, we can rewrite the equation as delta(U) = delta(H) - Pdelta(V). By the ideal gas law, PV = nRT, -PdeltaV = -delta(n)RT so we can rewrite the equation as delta(U) = delta(H) - delta(n)RT, which if you rearrange it, is delta(H) = delta(U) + delta(n)RT (the second equation you have there). Thus from this, we can see that the main difference between the two equations you listed is that one includes the change in internal energy of a system as a result of doing work. If no work is done (no change in volume of the system), then w = 0 and delta(U) = delta(H). It's not so much as disregarding delta(U) = delta(H) as knowing when you have done work.
-
lukezhang2C
- Posts: 59
- Joined: Fri Sep 28, 2018 12:18 am
Re: [tex]\Delta U = \Delta H[/tex]
So delta U = delta H is only considered a very specific case of the original equation, which is delta U = q + w = q - P delta V. You can only use delta U = delta H when q = delta H and you are under constant pressure, otherwise you cannot use the other equation.
Return to “Concepts & Calculations Using First Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 8 guests

