Calculating the Net Change in Moles of a Gas to find Delta V
Moderators: Chem_Mod, Chem_Admin
-
Shelby_Smallwood_2B
- Posts: 3
- Joined: Fri Sep 26, 2014 2:02 pm
Calculating the Net Change in Moles of a Gas to find Delta V
In homework 7.51, you need to calculate the change in volume using the ideal gas law. In order to do that, you must calculate the net change in gas moles (The balanced equation is given). How do you calculate the net change in the moles of gases in the reaction?
-
Niharika Reddy 1D
- Posts: 127
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Calculating the Net Change in Moles of a Gas to find Del
Net change in moles of gas=Δn=(moles of gas in the products)-(moles of gas in the reactants)
For this problem, there are 3 moles of gas in the products and 2 moles of gas in the reactants, so the net change in moles of gas is 3-2=1 mol.
For this problem, there are 3 moles of gas in the products and 2 moles of gas in the reactants, so the net change in moles of gas is 3-2=1 mol.
-
Shelby_Smallwood_2B
- Posts: 3
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Calculating the Net Change in Moles of a Gas to find Del
Thanks so much. Is it possible to have a negative net change in moles or would you do the absolute value?
-
Neil DSilva 1L
- Posts: 70
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Calculating the Net Change in Moles of a Gas to find Del
You can have a negative values. It follows the same sorta thinking as
values. It follows the same sorta thinking as  or
or 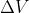 . It just means the change was a negative change and the final value is less than the initial value. So, for example, if there are 4 moles of gas in the reactants (instead of 2),
. It just means the change was a negative change and the final value is less than the initial value. So, for example, if there are 4 moles of gas in the reactants (instead of 2), 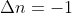 .
.
Return to “Concepts & Calculations Using First Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 7 guests

