Reversible and Irreversible Reactions
Moderators: Chem_Mod, Chem_Admin
-
ashwathinair
- Posts: 113
- Joined: Sat Aug 17, 2019 12:17 am
Reversible and Irreversible Reactions
I wasn't super sure where to put this, but I wanted to know how to tell the difference between reversible and irreversible reactions. Are phase changes reversible? What are the markers to know whether a chemical reaction is reversible or irreversible?
-
Eesha Sohail 1D
- Posts: 100
- Joined: Sat Aug 17, 2019 12:16 am
Re: Reversible and Irreversible Reactions
Physical changes involving a change in state are all reversible. For chemical reactions, I'm not sure exactly what markers you could look for to deem a reaction reversible, but it is useful to remember that reversibility is usually conditional, and reactions deemed irreversible may actually be reversible in certain industrial conditions.
-
Sukanya Mohapatra 2G
- Posts: 100
- Joined: Sat Aug 17, 2019 12:18 am
- Been upvoted: 1 time
Re: Reversible and Irreversible Reactions
In a irreversible reaction, the reactants react to form the products, which cannot revert back into reactants. In reversible reactions, as the reactants react with other reactants to form products, the products are reacting with other products to form reactants.
Re: Reversible and Irreversible Reactions
The lines are blurry, but generally you can call a reaction irreversible if it uses up all (or a considerable amount of) the reaction materials in its reaction, such as an explosion.
I'm not sure if you'll usually be told, but so long as the conditions of the reaction environment means that it can't go back, then the reaction is irreversible.
A good example of a reversible reaction that I like to keep in my mind is an acid-base reaction. Since those reactions thrive off of a constant cycle of association and dissociation in solution, we even write them using the symbol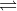 , showing that we know the reaction is going backwards to some extent.
, showing that we know the reaction is going backwards to some extent.
If the materials are still sitting in their reaction vessel and have the capacity to do work to reverse the reaction, then it's likely a reversible reaction
I'm not sure if you'll usually be told, but so long as the conditions of the reaction environment means that it can't go back, then the reaction is irreversible.
A good example of a reversible reaction that I like to keep in my mind is an acid-base reaction. Since those reactions thrive off of a constant cycle of association and dissociation in solution, we even write them using the symbol
If the materials are still sitting in their reaction vessel and have the capacity to do work to reverse the reaction, then it's likely a reversible reaction
Return to “Concepts & Calculations Using First Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 3 guests

