Change in Internal Energy Formula
Moderators: Chem_Mod, Chem_Admin
-
Jordi M 2I
- Posts: 152
- Joined: Wed Sep 30, 2020 9:40 pm
Change in Internal Energy Formula
In the lecture today Dr. Lavelle substituted work as the function 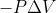 . Does this mean we can substitute in the formula for a reversible reaction under conditions of constant pressure? The one with the integral.
. Does this mean we can substitute in the formula for a reversible reaction under conditions of constant pressure? The one with the integral.
-
Andrew Jubintoro 3J
- Posts: 123
- Joined: Wed Sep 30, 2020 9:58 pm
- Been upvoted: 1 time
Re: Change in Internal Energy Formula
Yes, I believe you would use the appropriate formula for w.
In the case of a reversible expansion, you would use the one with the integral. Also, the pressure is not constant for reversible expansion.
In the case of a reversible expansion, you would use the one with the integral. Also, the pressure is not constant for reversible expansion.
-
isha dis3d
- Posts: 106
- Joined: Wed Sep 30, 2020 9:47 pm
Re: Change in Internal Energy Formula
The -P delta V equation is used when volume changes in an irreversible reaction, where the external pressure is constant I believe.
-
Pranav Kadiyala 1A
- Posts: 134
- Joined: Wed Sep 30, 2020 9:58 pm
Re: Change in Internal Energy Formula
I believe the overarching formula for work is ∫P*dV. (imagine v1 and v2 on the bottom and top of the integral, respectively). Since pressure is constant in an irreversible rxn (it's not changing, which is why it is less efficient) we can take it out of the equation. P*∫dv (again imagine v1 and v2). ∫dv is just v1-v2 = delta v. Since pressure is not constant in reversible rxns, we can's use this formula and stick with the integral.
Return to “Concepts & Calculations Using First Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 1 guest

