Since q=0 in an adiabatic system, is the entropy of an adiabatic system constant?
Thanks
change in entropy for adiabatic systems
Moderators: Chem_Mod, Chem_Admin
-
Estefania Lima 2G
- Posts: 14
- Joined: Fri Sep 25, 2015 3:00 am
-
Albert Chong_1L
- Posts: 77
- Joined: Fri Sep 25, 2015 3:00 am
Re: change in entropy for adiabatic systems
Factors other than heat flow (q) can affect entropy. For example, if volume was increased for a gas, there would be more entropy since the gas molecules have more possible micro states in which to exist.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
-
Albert Chong_1L
- Posts: 77
- Joined: Fri Sep 25, 2015 3:00 am
Re: change in entropy for adiabatic systems
Chem_Mod wrote:For reversible adiabatic process, delta S should be 0.
I can see that is true through deltaS=q/T, since deltaS will be 0 when q is 0.
However, if V2/V1 can be substituted for T2/T1 in the equation deltaS=nCln(T2/T1), then how does this equation show deltaS as 0 when q=0 (adiabatic)?
Is this equation only for free expansion in which entropy increases despite no change in internal energy?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: change in entropy for adiabatic systems
PV=nRT does not work for adiabatic process. It is in fact 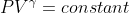 which is not covered in this class. For free expansion, delta S=nRln(V2/V1) due to the statistical model you have been shown in class (derived from delta S=nRln(W2/W1)
which is not covered in this class. For free expansion, delta S=nRln(V2/V1) due to the statistical model you have been shown in class (derived from delta S=nRln(W2/W1)
Return to “Concepts & Calculations Using Second Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 6 guests

