HW Problem 9.13 - Number of Moles
Moderators: Chem_Mod, Chem_Admin
-
Natalie Rotstein 3J
- Posts: 20
- Joined: Wed Sep 21, 2016 2:59 pm
HW Problem 9.13 - Number of Moles
I was working on problem 9.13, and I initially was going to use the equations ∆S=nRln(T2/T1) and ∆S=nRln(V2/V1), because they seemed appropriate, but I ended up stuck because they did not give the number of moles, and I didn't see any way to calculate it. I then went to the answer manual to figure out what at least the initial step was, and they used those equations but with n=1 mole. Is there a reason why one can assume that there is one mole, or a way to calculate it from the information given?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: HW Problem 9.13 - Number of Moles
There is no way to calculate the number of moles with the information given in the problem.
For problems in Chem 14B, you can assume gases have ideal behavior and use the ideal gas equation, PV = nRT, where P is pressure in atm, V is volume in liters, n is number of moles, R is the ideal gas constant: 0.08206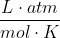 , and T is temperature in Kelvin, in order to solve for one of the missing variables. However, to find an unknown variable, you need to be given the values of all the other variables, so to find the number of moles (n), you would need to be given P, V, and T. Remember that R is a constant so its value always stays the same. For this problem, you are given T and V, but not P, so you cannot solve for n. That is why the solution manual tells you to assume 1 mol of
, and T is temperature in Kelvin, in order to solve for one of the missing variables. However, to find an unknown variable, you need to be given the values of all the other variables, so to find the number of moles (n), you would need to be given P, V, and T. Remember that R is a constant so its value always stays the same. For this problem, you are given T and V, but not P, so you cannot solve for n. That is why the solution manual tells you to assume 1 mol of 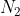 gas.
gas.
For problems in Chem 14B, you can assume gases have ideal behavior and use the ideal gas equation, PV = nRT, where P is pressure in atm, V is volume in liters, n is number of moles, R is the ideal gas constant: 0.08206
Return to “Concepts & Calculations Using Second Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 10 guests

