Spontaneous [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Ju-Wei Wang 1I
- Posts: 31
- Joined: Fri Sep 29, 2017 7:05 am
Spontaneous
In a problem, how are we supposed to determine whether a reaction/process is spontaneous or not? I know you can figure it out when given Gibbs free energy, but what other way can be used to figure this out?
-
Varsha Sivaganesh 1A
- Posts: 51
- Joined: Thu Jul 13, 2017 3:00 am
Re: Spontaneous
Well in class, Lavelle mentioned that in any spontaneous process, there is an increase in entropy. However, I'm not sure if that means ALL spontaneous processes have a positive 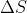
-
Susie Lee 2I
- Posts: 20
- Joined: Fri Sep 29, 2017 7:05 am
-
Diane Bui 2J
- Posts: 61
- Joined: Sat Jul 22, 2017 3:00 am
Re: Spontaneous
A spontaneous process means that it has the tendency to occur naturally without an outside force acting on it.
-
Tiffany 1B
- Posts: 32
- Joined: Fri Sep 29, 2017 7:05 am
Re: Spontaneous
Yeah so with spontaneous processes the entropy does increase so I think you would probably be able to assume that a positive delta S means spontaneous. Although Gibbs free energy might be a more definite way...
-
Felicia Fong 2G
- Posts: 31
- Joined: Sat Jul 22, 2017 3:00 am
Re: Spontaneous
An example for a spontaneous process would be a ball falling down a hill. This occurs naturally. A non spontaneous process would be a ball rolling uphill, which wouldn't occur on its own.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Spontaneous [ENDORSED]
Spontaneity is dependent on the value of the Gibb's free energy for a process. Spontaneous processes will have a negative value. Just because a process is exothermic or increases entropy does not mean that it is spontaneous. For example, melting ice increases entropy, but ice will not melt below 0 C.
Return to “Concepts & Calculations Using Second Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 9 guests

