Celsius or Kelvin
Moderators: Chem_Mod, Chem_Admin
-
Abigail Urbina 1K
- Posts: 102
- Joined: Thu Jul 27, 2017 3:01 am
Celsius or Kelvin
If asked to calculate the changes in entropy and we are given temperature in terms of Celsius, do we always have to convert the temperature to be in terms of Kelvin? I know both can technically be considered correct, but is there a preferred method?
-
Kevin Ru 1D
- Posts: 50
- Joined: Thu Jul 13, 2017 3:00 am
Re: Celsius or Kelvin
You should convert to Kelvin as it is no longer ΔT in the equation, but T (The general formula for change in entropy being ΔS = qrev/T).
Re: Celsius or Kelvin
When in doubt, it is always best to convert the temperature to Kelvin by adding 273.15.
-
Jason Liu 1C
- Posts: 52
- Joined: Fri Sep 29, 2017 7:04 am
Re: Celsius or Kelvin
For problems with ΔT, you can use either Celsius or Kelvin because the difference between the temperatures will be the same. However, for problems with just T, you should generally use Kelvin because that is the SI unit.
-
Erica Nagase 1H
- Posts: 30
- Joined: Sat Jul 22, 2017 3:00 am
Re: Celsius or Kelvin
For problems with ln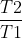 , temperature has to be in K because the conversion factor is additive (+273) and won't cancel out. In comparison, the units don't really matter for problems with ln
, temperature has to be in K because the conversion factor is additive (+273) and won't cancel out. In comparison, the units don't really matter for problems with ln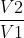 or ln
or ln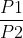 because the conversion factors are multiplied (like x1000mL) and cancel out.
because the conversion factors are multiplied (like x1000mL) and cancel out.
-
Cynthia Bui 2H
- Posts: 30
- Joined: Fri Sep 29, 2017 7:05 am
Return to “Concepts & Calculations Using Second Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 13 guests

