∆S=q/T
Moderators: Chem_Mod, Chem_Admin
-
Sarah_Stay_1D
- Posts: 57
- Joined: Sat Jul 22, 2017 3:00 am
- Been upvoted: 1 time
Re: ∆S=q/T
Ya Gao wrote:Can someone explain why ∆S=q/T=nR ln(V2/V1)? I know that ∆S=klnW2/W1, so does nR equals k then?
I cannot recall whether Lavelle went through in lecture how to derive the equation ∆S=nRln(V2/V1), however it is simply another way to calculate the entropy of the system. However this equation allows you to look at a system that is changing in volume. The other formula you are referring to is not ∆S=klnW2?W1. I believe what you are referring to is S=KblnW (where Kb is the Boltzmann constant, and W is the degeneracy, also notice it is just S, not ∆S). The variable k typically represents the equilibrium constant which is different for different reactions. You can find the equilibrium constant using the equation either the way we learned in 14A, or using the equation ∆G=-RTlnK.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: ∆S=q/T
For the first derivation we get,
}{T} = \frac{P_{ext}dV}{T})
We get this from reversible work being done by an isothermal system (ie- T is constant,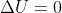 , q=-w).
, q=-w).
Now we use the substitution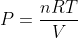 to get
to get
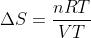 and notice the temperature cancels.
and notice the temperature cancels.
So,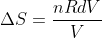
Last thing is to integrate and use a law of logs to acheive our derived equation,
 - ln(V_{i})) = nRln(\frac{V_{f}}{V_{i}}))
You can find this derivation and a useful blurb in your text book on page 323.
We get this from reversible work being done by an isothermal system (ie- T is constant,
Now we use the substitution
So,
Last thing is to integrate and use a law of logs to acheive our derived equation,
You can find this derivation and a useful blurb in your text book on page 323.
Return to “Concepts & Calculations Using Second Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 13 guests

